TASK-1 Regulates Apoptosis and Proliferation in a Subset of Non-Small Cell Lung Cancers
- PMID: 27294516
- PMCID: PMC4905626
- DOI: 10.1371/journal.pone.0157453
TASK-1 Regulates Apoptosis and Proliferation in a Subset of Non-Small Cell Lung Cancers
Abstract
Lung cancer is the leading cause of cancer deaths worldwide; survival times are poor despite therapy. The role of the two-pore domain K+ (K2P) channel TASK-1 (KCNK3) in lung cancer is at present unknown. We found that TASK-1 is expressed in non-small cell lung cancer (NSCLC) cell lines at variable levels. In a highly TASK-1 expressing NSCLC cell line, A549, a characteristic pH- and hypoxia-sensitive non-inactivating K+ current was measured, indicating the presence of functional TASK-1 channels. Inhibition of TASK-1 led to significant depolarization in these cells. Knockdown of TASK-1 by siRNA significantly enhanced apoptosis and reduced proliferation in A549 cells, but not in weakly TASK-1 expressing NCI-H358 cells. Na+-coupled nutrient transport across the cell membrane is functionally coupled to the efflux of K+ via K+ channels, thus TASK-1 may potentially influence Na+-coupled nutrient transport. In contrast to TASK-1, which was not differentially expressed in lung cancer vs. normal lung tissue, we found the Na+-coupled nutrient transporters, SLC5A3, SLC5A6, and SLC38A1, transporters for myo-inositol, biotin and glutamine, respectively, to be significantly overexpressed in lung adenocarcinomas. In summary, we show for the first time that the TASK-1 channel regulates apoptosis and proliferation in a subset of NSCLC.
Conflict of interest statement
Figures

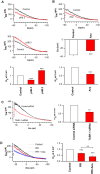
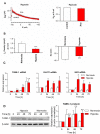
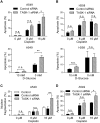
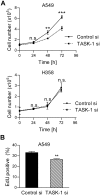

References
-
- Manegold C, Thatcher N. Survival improvement in thoracic cancer: progress from the last decade and beyond. Lung Cancer. 2007;57 Suppl 2:S3–S5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

