S6K1 regulates hematopoietic stem cell self-renewal and leukemia maintenance
- PMID: 27294524
- PMCID: PMC4922705
- DOI: 10.1172/JCI84565
S6K1 regulates hematopoietic stem cell self-renewal and leukemia maintenance
Abstract
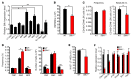
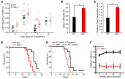
Hyperactivation of the mTOR pathway impairs hematopoietic stem cell (HSC) functions and promotes leukemogenesis. mTORC1 and mTORC2 differentially control normal and leukemic stem cell functions. mTORC1 regulates p70 ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding (eIF4E-binding) protein 1 (4E-BP1), and mTORC2 modulates AKT activation. Given the extensive crosstalk that occurs between mTORC1 and mTORC2 signaling pathways, we assessed the role of the mTORC1 substrate S6K1 in the regulation of both normal HSC functions and in leukemogenesis driven by the mixed lineage leukemia (MLL) fusion oncogene MLL-AF9. We demonstrated that S6K1 deficiency impairs self-renewal of murine HSCs by reducing p21 expression. Loss of S6K1 also improved survival in mice transplanted with MLL-AF9-positive leukemic stem cells by modulating AKT and 4E-BP1 phosphorylation. Taken together, these results suggest that S6K1 acts through multiple targets of the mTOR pathway to promote self-renewal and leukemia progression. Given the recent interest in S6K1 as a potential therapeutic target in cancer, our results further support targeting this molecule as a potential strategy for treatment of myeloid malignancies.
Figures

Similar articles
-
Role of mTORC1-S6K1 signaling pathway in regulation of hematopoietic stem cell and acute myeloid leukemia.Exp Hematol. 2017 Jun;50:13-21. doi: 10.1016/j.exphem.2017.02.004. Epub 2017 Mar 22. Exp Hematol. 2017. PMID: 28342808 Free PMC article. Review.
-
Rapamycin attenuates BAFF-extended proliferation and survival via disruption of mTORC1/2 signaling in normal and neoplastic B-lymphoid cells.J Cell Physiol. 2018 Jan;233(1):516-529. doi: 10.1002/jcp.25913. Epub 2017 May 3. J Cell Physiol. 2018. PMID: 28300280 Free PMC article.
-
Both mTORC1 and mTORC2 are involved in the regulation of cell adhesion.Oncotarget. 2015 Mar 30;6(9):7136-50. doi: 10.18632/oncotarget.3044. Oncotarget. 2015. PMID: 25762619 Free PMC article.
-
S6K1 alternative splicing modulates its oncogenic activity and regulates mTORC1.Cell Rep. 2013 Jan 31;3(1):103-15. doi: 10.1016/j.celrep.2012.11.020. Epub 2012 Dec 27. Cell Rep. 2013. PMID: 23273915 Free PMC article.
-
The complexes of mammalian target of rapamycin.Curr Protein Pept Sci. 2010 Sep;11(6):409-24. doi: 10.2174/138920310791824093. Curr Protein Pept Sci. 2010. PMID: 20491627 Free PMC article. Review.
Cited by
-
A structured population model of clonal selection in acute leukemias with multiple maturation stages.J Math Biol. 2019 Oct;79(5):1587-1621. doi: 10.1007/s00285-019-01404-w. Epub 2019 Jul 26. J Math Biol. 2019. PMID: 31350582
-
Role of mTORC1-S6K1 signaling pathway in regulation of hematopoietic stem cell and acute myeloid leukemia.Exp Hematol. 2017 Jun;50:13-21. doi: 10.1016/j.exphem.2017.02.004. Epub 2017 Mar 22. Exp Hematol. 2017. PMID: 28342808 Free PMC article. Review.
-
Beyond controlling cell size: functional analyses of S6K in tumorigenesis.Cell Death Dis. 2022 Jul 25;13(7):646. doi: 10.1038/s41419-022-05081-4. Cell Death Dis. 2022. PMID: 35879299 Free PMC article. Review.
-
Ribosomal protein L34 promotes the proliferation, invasion and metastasis of pancreatic cancer cells.Oncotarget. 2016 Dec 20;7(51):85259-85272. doi: 10.18632/oncotarget.13269. Oncotarget. 2016. PMID: 27845896 Free PMC article.
-
CD37 regulates the self-renewal of leukemic stem cells via integrin-mediated signaling in acute myeloid leukemia.Stem Cell Reports. 2025 May 13;20(5):102476. doi: 10.1016/j.stemcr.2025.102476. Epub 2025 Apr 17. Stem Cell Reports. 2025. PMID: 40250439 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

