Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality
- PMID: 27294527
- PMCID: PMC4922691
- DOI: 10.1172/JCI85796
Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality
Abstract
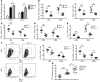
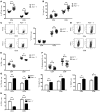
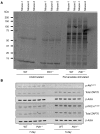
Programmed death ligand-1 (PD-L1) interaction with PD-1 induces T cell exhaustion and is a therapeutic target to enhance immune responses against cancer and chronic infections. In murine bone marrow transplant models, PD-L1 expression on host target tissues reduces the incidence of graft-versus-host disease (GVHD). PD-L1 is also expressed on T cells; however, it is unclear whether PD-L1 on this population influences immune function. Here, we examined the effects of PD-L1 modulation of T cell function in GVHD. In patients with severe GVHD, PD-L1 expression was increased on donor T cells. Compared with mice that received WT T cells, GVHD was reduced in animals that received T cells from Pdl1-/- donors. PD-L1-deficient T cells had reduced expression of gut homing receptors, diminished production of inflammatory cytokines, and enhanced rates of apoptosis. Moreover, multiple bioenergetic pathways, including aerobic glycolysis, oxidative phosphorylation, and fatty acid metabolism, were also reduced in T cells lacking PD-L1. Finally, the reduction of acute GVHD lethality in mice that received Pdl1-/- donor cells did not affect graft-versus-leukemia responses. These data demonstrate that PD-L1 selectively enhances T cell-mediated immune responses, suggesting a context-dependent function of the PD-1/PD-L1 axis, and suggest selective inhibition of PD-L1 on donor T cells as a potential strategy to prevent or ameliorate GVHD.
Figures








References
-
- Sheppard KA, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1–3):37–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 CA120780/CA/NCI NIH HHS/United States
- R01 HL095791/HL/NHLBI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- R01 HL118979/HL/NHLBI NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- R01 AI043542/AI/NIAID NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- R37 AI038310/AI/NIAID NIH HHS/United States
- R01 CA103320/CA/NCI NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- R35 CA197292/CA/NCI NIH HHS/United States
- R37 AI043542/AI/NIAID NIH HHS/United States
- T32 HL007775/HL/NHLBI NIH HHS/United States
- R37 HL056067/HL/NHLBI NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- R01 CA072669/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

