Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation
- PMID: 27294875
- PMCID: PMC5004598
- DOI: 10.1038/nm.4124
Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation
Abstract
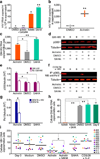
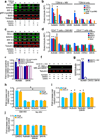
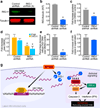
The persistence of latent HIV proviruses in long-lived CD4(+) T cells despite antiretroviral therapy (ART) is a major obstacle to viral eradication. Because current candidate latency-reversing agents (LRAs) induce HIV transcription, but fail to clear these cellular reservoirs, new approaches for killing these reactivated latent HIV reservoir cells are urgently needed. HIV latency depends upon the transcriptional quiescence of the integrated provirus and the circumvention of immune defense mechanisms. These defenses include cell-intrinsic innate responses that use pattern-recognition receptors (PRRs) to detect viral pathogens, and that subsequently induce apoptosis of the infected cell. Retinoic acid (RA)-inducible gene I (RIG-I, encoded by DDX58) forms one class of PRRs that mediates apoptosis and the elimination of infected cells after recognition of viral RNA. Here we show that acitretin, an RA derivative approved by the US Food and Drug Administration (FDA), enhances RIG-I signaling ex vivo, increases HIV transcription, and induces preferential apoptosis of HIV-infected cells. These effects are abrogated by DDX58 knockdown. Acitretin also decreases proviral DNA levels in CD4(+) T cells from HIV-positive subjects on suppressive ART, an effect that is amplified when combined with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor. Pharmacological enhancement of an innate cellular-defense network could provide a means by which to eliminate reactivated cells in the latent HIV reservoir.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. - PubMed
-
- Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. - PubMed
-
- Passaes CP, Saez-Cirion A. HIV cure research: advances and prospects. Virology. 2014;454–455:340–352. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

