Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan
- PMID: 27295191
- PMCID: PMC4904795
- DOI: 10.1038/srep28039
Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan
Abstract
Tumour blood vessels are gateways for distant metastasis. Recent studies have revealed that tumour endothelial cells (TECs) demonstrate distinct phenotypes from their normal counterparts. We have demonstrated that features of TECs are different depending on tumour malignancy, suggesting that TECs communicate with surrounding tumour cells. However, the contribution of TECs to metastasis has not been elucidated. Here, we show that TECs actively promote tumour metastasis through a bidirectional interaction between tumour cells and TECs. Co-implantation of TECs isolated from highly metastatic tumours accelerated lung metastases of low metastatic tumours. Biglycan, a small leucine-rich repeat proteoglycan secreted from TECs, activated tumour cell migration via nuclear factor-κB and extracellular signal-regulated kinase 1/2. Biglycan expression was upregulated by DNA demethylation in TECs. Collectively, our results demonstrate that TECs are altered in their microenvironment and, in turn, instigate tumour cells to metastasize, which is a novel mechanism for tumour metastasis.
Figures
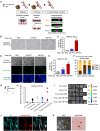
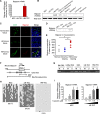
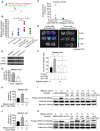
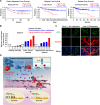
References
-
- Chambers A. F., Groom A. C. & MacDonald I. C. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2, 563–572 (2002). - PubMed
-
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29, 15–18 (2002). - PubMed
-
- Weidner N., Semple J. P., Welch W. R. & Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324, 1–8 (1991). - PubMed
-
- Fidler I. J. Angiogenic heterogeneity: regulation of neoplastic angiogenesis by the organ microenvironment. J Natl Cancer Inst 93, 1040–1041 (2001). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

