Molecular Dynamics Study of Twister Ribozyme: Role of Mg(2+) Ions and the Hydrogen-Bonding Network in the Active Site
- PMID: 27295275
- PMCID: PMC5127262
- DOI: 10.1021/acs.biochem.6b00203
Molecular Dynamics Study of Twister Ribozyme: Role of Mg(2+) Ions and the Hydrogen-Bonding Network in the Active Site
Abstract
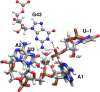
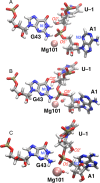
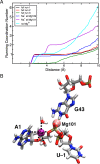
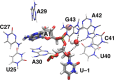
The recently discovered twister ribozyme is thought to utilize general acid-base catalysis in its self-cleavage mechanism, but the roles of nucleobases and metal ions in the mechanism are unclear. Herein, molecular dynamics simulations of the env22 twister ribozyme are performed to elucidate the structural and equilibrium dynamical properties, as well as to examine the role of Mg(2+) ions and possible candidates for the general base and acid in the self-cleavage mechanism. The active site region and the ends of the pseudoknots were found to be less mobile than other regions of the ribozyme, most likely providing structural stability and possibly facilitating catalysis. A purported catalytic Mg(2+) ion and the closest neighboring Mg(2+) ion remained chelated and relatively immobile throughout the microsecond trajectories, although removal of these Mg(2+) ions did not lead to any significant changes in the structure or equilibrium motions of the ribozyme on the microsecond time scale. In addition, a third metal ion, a Na(+) ion remained close to A1(O5'), the leaving group atom, during the majority of the microsecond trajectories, suggesting that it might stabilize the negative charge on A1(O5') during self-cleavage. The locations of these cations and their interactions with key nucleotides in the active site suggest that they may be catalytically relevant. The P1 stem is partially melted at its top and bottom in the crystal structure and further unwinds in the trajectories. The simulations also revealed an interconnected network comprised of hydrogen-bonding and π-stacking interactions that create a relatively rigid network around the self-cleavage site. The nucleotides involved in this network are among the highly conserved nucleotides in twister ribozymes, suggesting that this interaction network may be important to structure and function.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Wobble pairs of the HDV ribozyme play specific roles in stabilization of active site dynamics.Phys Chem Chem Phys. 2015 Feb 28;17(8):5887-900. doi: 10.1039/c4cp05083e. Phys Chem Chem Phys. 2015. PMID: 25631765 Free PMC article.
-
Structural dynamics of precursor and product of the RNA enzyme from the hepatitis delta virus as revealed by molecular dynamics simulations.J Mol Biol. 2005 Aug 26;351(4):731-48. doi: 10.1016/j.jmb.2005.06.016. J Mol Biol. 2005. PMID: 16045932
-
Mechanistic strategies in the HDV ribozyme: chelated and diffuse metal ion interactions and active site protonation.J Phys Chem B. 2011 Jun 30;115(25):8346-57. doi: 10.1021/jp203202e. Epub 2011 Jun 7. J Phys Chem B. 2011. PMID: 21644800 Free PMC article.
-
Two distinct catalytic strategies in the hepatitis δ virus ribozyme cleavage reaction.Biochemistry. 2011 Nov 8;50(44):9424-33. doi: 10.1021/bi201157t. Epub 2011 Oct 17. Biochemistry. 2011. PMID: 22003985 Free PMC article. Review.
-
Structural and Biochemical Properties of Novel Self-Cleaving Ribozymes.Molecules. 2017 Apr 24;22(4):678. doi: 10.3390/molecules22040678. Molecules. 2017. PMID: 28441772 Free PMC article. Review.
Cited by
-
Elucidation of Catalytic Strategies of Small Nucleolytic Ribozymes From Comparative Analysis of Active Sites.ACS Catal. 2018 Jan 5;8(1):314-327. doi: 10.1021/acscatal.7b02976. Epub 2017 Dec 8. ACS Catal. 2018. PMID: 32547833 Free PMC article.
-
The effect of adenine protonation on RNA phosphodiester backbone bond cleavage elucidated by deaza-nucleobase modifications and mass spectrometry.Nucleic Acids Res. 2019 Aug 22;47(14):7223-7234. doi: 10.1093/nar/gkz574. Nucleic Acids Res. 2019. PMID: 31276590 Free PMC article.
-
High-Throughput Mutational Analysis of a Twister Ribozyme.Angew Chem Int Ed Engl. 2016 Aug 22;55(35):10354-7. doi: 10.1002/anie.201605470. Epub 2016 Jul 27. Angew Chem Int Ed Engl. 2016. PMID: 27461281 Free PMC article.
-
Mg2+ Impacts the Twister Ribozyme through Push-Pull Stabilization of Nonsequential Phosphate Pairs.Biophys J. 2020 Mar 24;118(6):1424-1437. doi: 10.1016/j.bpj.2020.01.021. Epub 2020 Jan 28. Biophys J. 2020. PMID: 32053774 Free PMC article.
-
1-Deazaguanosine-Modified RNA: The Missing Piece for Functional RNA Atomic Mutagenesis.J Am Chem Soc. 2022 Jun 15;144(23):10344-10352. doi: 10.1021/jacs.2c01877. Epub 2022 Jun 6. J Am Chem Soc. 2022. PMID: 35666572 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

