Ecophysiological consequences of alcoholism on human gut microbiota: implications for ethanol-related pathogenesis of colon cancer
- PMID: 27295340
- PMCID: PMC4904738
- DOI: 10.1038/srep27923
Ecophysiological consequences of alcoholism on human gut microbiota: implications for ethanol-related pathogenesis of colon cancer
Abstract
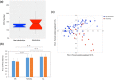
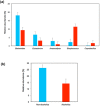
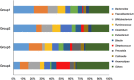
Chronic consumption of excess ethanol increases the risk of colorectal cancer. The pathogenesis of ethanol-related colorectal cancer (ER-CRC) is thought to be partly mediated by gut microbes. Specifically, bacteria in the colon and rectum convert ethanol to acetaldehyde (AcH), which is carcinogenic. However, the effects of chronic ethanol consumption on the human gut microbiome are poorly understood, and the role of gut microbes in the proposed AcH-mediated pathogenesis of ER-CRC remains to be elaborated. Here we analyse and compare the gut microbiota structures of non-alcoholics and alcoholics. The gut microbiotas of alcoholics were diminished in dominant obligate anaerobes (e.g., Bacteroides and Ruminococcus) and enriched in Streptococcus and other minor species. This alteration might be exacerbated by habitual smoking. These observations could at least partly be explained by the susceptibility of obligate anaerobes to reactive oxygen species, which are increased by chronic exposure of the gut mucosa to ethanol. The AcH productivity from ethanol was much lower in the faeces of alcoholic patients than in faeces of non-alcoholic subjects. The faecal phenotype of the alcoholics could be rationalised based on their gut microbiota structures and the ability of gut bacteria to accumulate AcH from ethanol.
Figures



References
-
- Mizoue T. et al. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol 167, 1397–1406 (2008). - PubMed
-
- Seitz H. K. & Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 7, 599–612 (2007). - PubMed
-
- Mizukami T., Yokoyama A., Maruyama K. & Hibi T. Screening colonoscopy for Japanese alcoholic men (in Japanese). Shokakika 48, 599–604 (2009).
-
- Salaspuro M. Microbial metabolism of ethanol and acetaldehyde and clinical consequences. Addiction Biol 2, 35–46 (1997). - PubMed
-
- Das S. K. & Vasudevan D. M. Alcohol-induced oxidative stress. Life Sci 81, 177–187 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

