Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum
- PMID: 27295972
- PMCID: PMC4906402
- DOI: 10.1038/srep27956
Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum
Abstract
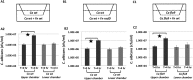
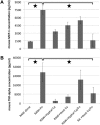
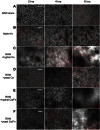
Candida albicans and Fusobacterium nucleatum are well-studied oral commensal microbes with pathogenic potential that are involved in various oral polymicrobial infectious diseases. Recently, we demonstrated that F. nucleatum ATCC 23726 coaggregates with C. albicans SN152, a process mainly mediated by fusobacterial membrane protein RadD and Candida cell wall protein Flo9. The aim of this study was to investigate the potential biological impact of this inter-kingdom interaction. We found that F. nucleatum ATCC 23726 inhibits growth and hyphal morphogenesis of C. albicans SN152 in a contact-dependent manner. Further analysis revealed that the inhibition of Candida hyphal morphogenesis is mediated via RadD and Flo9 protein pair. Using a murine macrophage cell line, we showed that the F. nucleatum-induced inhibition of Candida hyphal morphogenesis promotes C. albicans survival and negatively impacts the macrophage-killing capability of C. albicans. Furthermore, the yeast form of C. albicans repressed F. nucleatum-induced MCP-1 and TNFα production in macrophages. Our study suggests that the interaction between C. albicans and F. nucleatum leads to a mutual attenuation of virulence, which may function to promote a long-term commensal lifestyle within the oral cavity. This finding has significant implications for our understanding of inter-kingdom interaction and may impact clinical treatment strategies.
Figures



Similar articles
-
Cellular Components Mediating Coadherence of Candida albicans and Fusobacterium nucleatum.J Dent Res. 2015 Oct;94(10):1432-8. doi: 10.1177/0022034515593706. Epub 2015 Jul 7. J Dent Res. 2015. PMID: 26152186 Free PMC article.
-
Coaggregation of Candida dubliniensis with Fusobacterium nucleatum.J Clin Microbiol. 1999 May;37(5):1464-8. doi: 10.1128/JCM.37.5.1464-1468.1999. J Clin Microbiol. 1999. PMID: 10203506 Free PMC article.
-
Candida albicans morphogenesis is not required for macrophage interleukin 1β production.mBio. 2012 Dec 26;4(1):e00433-12. doi: 10.1128/mBio.00433-12. mBio. 2012. PMID: 23269828 Free PMC article.
-
Fusobacterium nucleatum - symbiont, opportunist and oncobacterium.Nat Rev Microbiol. 2019 Mar;17(3):156-166. doi: 10.1038/s41579-018-0129-6. Nat Rev Microbiol. 2019. PMID: 30546113 Free PMC article. Review.
-
Iron at the Centre of Candida albicans Interactions.Front Cell Infect Microbiol. 2018 Jun 5;8:185. doi: 10.3389/fcimb.2018.00185. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29922600 Free PMC article. Review.
Cited by
-
The Oral Bacterium Fusobacterium nucleatum Binds Staphylococcus aureus and Alters Expression of the Staphylococcal Accessory Regulator sarA.Microb Ecol. 2019 Aug;78(2):336-347. doi: 10.1007/s00248-018-1291-0. Epub 2018 Nov 24. Microb Ecol. 2019. PMID: 30474730 Free PMC article.
-
Pathogenetic Impact of Bacterial-Fungal Interactions.Microorganisms. 2019 Oct 16;7(10):459. doi: 10.3390/microorganisms7100459. Microorganisms. 2019. PMID: 31623187 Free PMC article. Review.
-
Fusobacterium Species and Subspecies Differentially Affect the Composition and Architecture of Supra- and Subgingival Biofilms Models.Front Microbiol. 2019 Jul 30;10:1716. doi: 10.3389/fmicb.2019.01716. eCollection 2019. Front Microbiol. 2019. PMID: 31417514 Free PMC article.
-
Fungi-A Component of the Oral Microbiome Involved in Periodontal Diseases.Adv Exp Med Biol. 2022;1373:113-138. doi: 10.1007/978-3-030-96881-6_6. Adv Exp Med Biol. 2022. PMID: 35612795
-
Bio- and Nanotechnology as the Key for Clinical Application of Salivary Peptide Histatin: A Necessary Advance.Microorganisms. 2020 Jul 10;8(7):1024. doi: 10.3390/microorganisms8071024. Microorganisms. 2020. PMID: 32664360 Free PMC article. Review.
References
-
- Paster B. J., Olsen I., Aas J. A. & Dewhirst F. E. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 42, 80–7 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

