Donor age and long-term culture do not negatively influence the stem potential of limbal fibroblast-like stem cells
- PMID: 27296060
- PMCID: PMC4906894
- DOI: 10.1186/s13287-016-0342-z
Donor age and long-term culture do not negatively influence the stem potential of limbal fibroblast-like stem cells
Erratum in
-
Erratum to: Donor age and long-term culture do not negatively influence the stem potential of limbal fibroblast-like stem cells.Stem Cell Res Ther. 2016 Aug 10;7(1):106. doi: 10.1186/s13287-016-0381-5. Stem Cell Res Ther. 2016. PMID: 27510649 Free PMC article. No abstract available.
Abstract
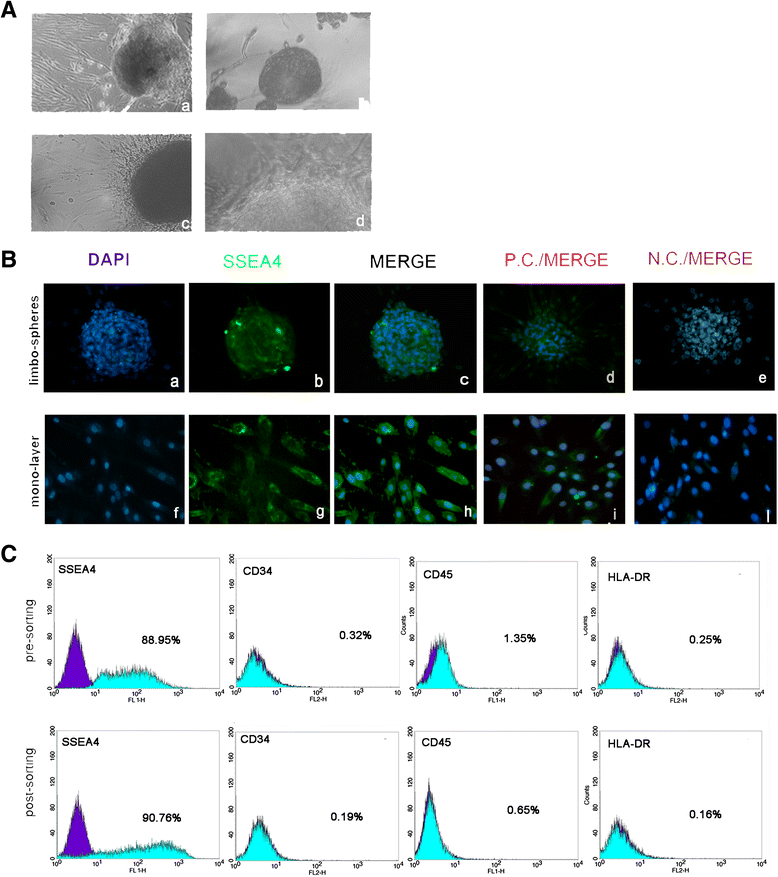
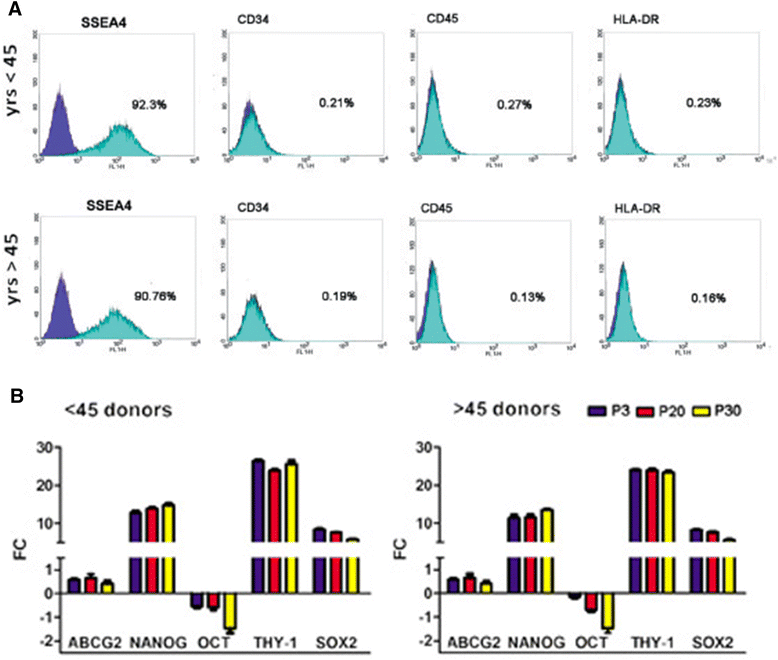
Background: In regenerative medicine the maintenance of stem cell properties is of crucial importance. Ageing is considered a cause of reduced stemness capability. The limbus is a stem niche of easy access and harbors two stem cell populations: epithelial stem cells and fibroblast-like stem cells. Our aim was to investigate whether donor age and/or long-term culture have any influence on stem cell marker expression and the profiles in the fibroblast-like stem cell population.
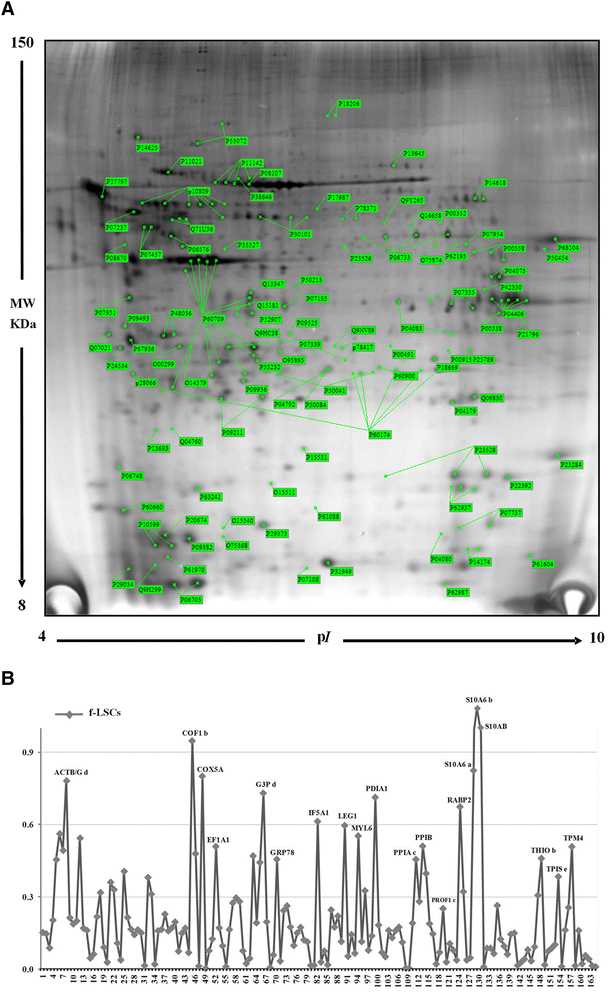
Methods: Fibroblast-like stem cells were isolated and digested from 25 limbus samples of normal human corneo-scleral rings and long-term cultures were obtained. SSEA4 expression and sphere-forming capability were evaluated; cytofluorimetric assay was performed to detect the immunophenotypes HLA-DR, CD45, and CD34 and the principle stem cell markers ABCG2, OCT3/4, and NANOG. Molecular expression of the principal mesenchymal stem cell genes was investigated by real-time PCR. Two-dimensional gel electrophoresis and mass spectrometric sequencing were performed and a stable proteomic profile was identified. The proteins detected were explored by gene ontology and STRING analysis. The data were reported as means ± SD, compared by Student's unpaired t test and considering p < 0.05 as statistically significant.
Results: The isolated cells did not display any hematopoietic surface marker (CD34 and CD45) and HLA-DR and they maintained these features in long-term culture. The expression of the stemness genes and the multilineage differentiation under in-vitro culture conditions proved to be well maintained. Proteomic analysis revealed a fibroblast-like stem cell profile of 164 proteins with higher expression levels. Eighty of these showed stable expression levels and were involved in maintenance of "the stem gene profile"; 84 were differentially expressed and were involved in structural activity.
Conclusions: The fibroblast-like limbal stem cells confirmed that they are a robust source of adult stem cells and that they have good plasticity, good proliferative capability, and long-term maintenance of stem cell properties, independently of donor age and long-term culture conditions. Our findings confirm that limbal fibroblast-like stem cells are highly promising for application in regenerative medicine and that in-vitro culture steps do not influence their stem cell properties. Moreover, the proteomic data enrich our knowledge of fibroblast-like stem cells.
Keywords: Adult stem cell pluripotency; Fibroblast-like stem cells; Limbal stem cells; Proteomic profile; Regenerative medicine.
Figures



References
-
- Schofield R. The stem cell system. Biomed Pharmacother. 1983;37:375–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

