Preconception Alcohol Increases Offspring Vulnerability to Stress
- PMID: 27296153
- PMCID: PMC5026748
- DOI: 10.1038/npp.2016.92
Preconception Alcohol Increases Offspring Vulnerability to Stress
Abstract
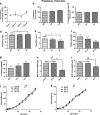
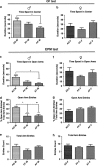
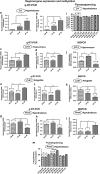
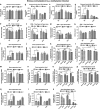
The effect of preconception drinking by the mother on the life-long health outcomes of her children is not known, and therefore, in this study using an animal model, we determined the impact of preconception alcohol drinking of the mother on offspring stress response during adulthood. In our preconception alcohol exposure model, adult female rats were fed with 6.7% alcohol in their diet for 4 weeks, went without alcohol for 3 weeks and were bred to generate male and female offspring. Preconception alcohol-exposed offsprings' birth weight, body growth, stress response, anxiety-like behaviors, and changes in stress regulatory gene and protein hormone levels were evaluated. In addition, roles of epigenetic mechanisms in preconception alcohol effects were determined. Alcohol feeding three weeks prior to conception significantly affected pregnancy outcomes of female rats, with respect to delivery period and birth weight of offspring, without affecting maternal care behaviors. Preconception alcohol negatively affected offspring adult health, producing an increased stress hormone response to an immune challenge. In addition, preconception alcohol was associated with changes in expression and methylation profiles of stress regulatory genes in various brain areas. These changes in stress regulatory genes were normalized following treatment with a DNA methylation blocker during the postnatal period. These data highlight the novel possibility that preconception alcohol affects the inheritance of stress-related diseases possibly by epigenetic mechanisms.
Figures

References
-
- Blizard DA, Lippman HR, Chen JJ (1975). Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav 14: 601–608. - PubMed
-
- Cameron N, Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K et al (2005). The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci Biobehav Rev 29: 843–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

