Clonorchis sinensis omega-class glutathione transferases play major roles in the protection of the reproductive system during maturation and the response to oxidative stress
- PMID: 27296469
- PMCID: PMC4906895
- DOI: 10.1186/s13071-016-1622-2
Clonorchis sinensis omega-class glutathione transferases play major roles in the protection of the reproductive system during maturation and the response to oxidative stress
Abstract
Background: Clonorchis sinensis causes a major food-borne helminthic infection. This species locates in mammalian hepatobiliary ducts, where oxidative stressors and hydrophobic substances are profuse. To adapt to the hostile micromilieu and to ensure its long-term survival, the parasite continuously produces a diverse repertoire of antioxidant enzymes including several species of glutathione transferases (GSTs). Helminth GSTs play pertinent roles during sequestration of harmful xenobiotics since most helminths lack the cytochrome P-450 detoxifying enzyme.
Methods: We isolated and analyzed the biochemical properties of two omega-class GSTs of C. sinensis (CsGSTo1 and CsGSTo2). We observed spatiotemporal expression patterns in accordance with the maturation of the worm's reproductive system. Possible biological protective roles of CsGSTos in these organs under oxidative stress were investigated.
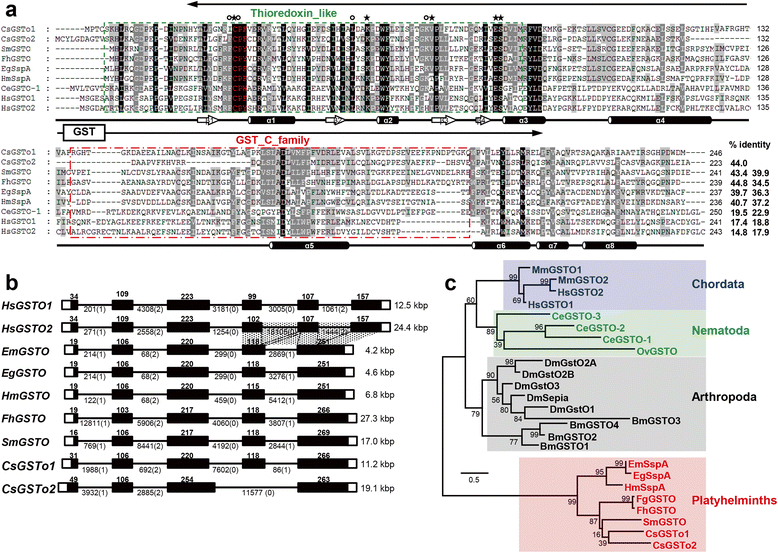
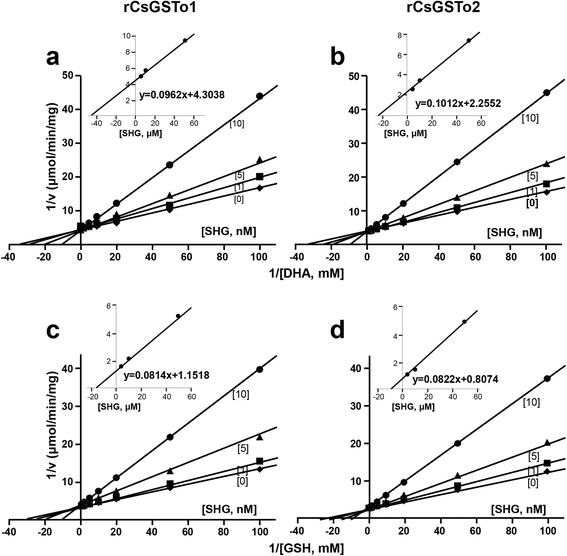
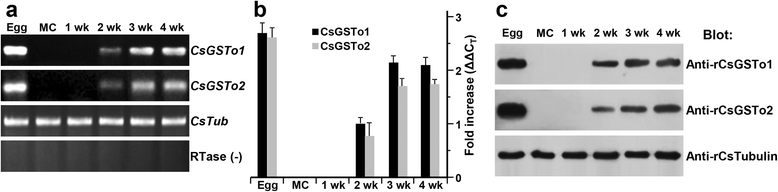
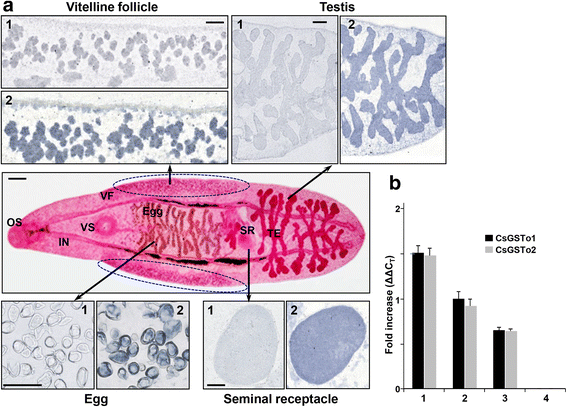
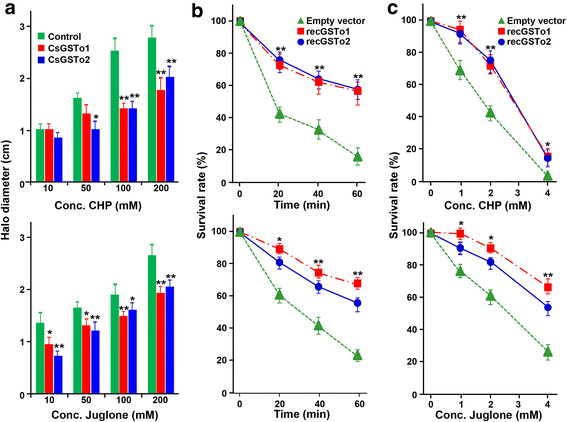
Results: The full-length cDNAs of CsGSTo1 and 2 constituted 965 bp and 1,061 bp with open reading frames of 737 bp (246 amino acids) and 669 bp (223 amino acids). They harbored characteristic N-terminal thioredoxin-like and C-terminal α-helical domains. A cysteine residue, which constituted omega-class specific active site, and the glutathione-binding amino acids, were recognized in appropriate positions. They shared 44 % sequence identity with each other and 14.8-44.8 % with orthologues/homologues from other organisms. Bacterially expressed recombinant proteins (rCsGSTo1 and 2) exhibited dehydroascorbate reductase (DHAR) and thioltransferase activities. DHAR activity was higher than thioltransferase activity. They showed weak canonical GST activity toward 1-chloro-2,4-dinitrobenzene. S-hexylglutathione potently and competitively inhibited the active-site at nanomolar concentrations (0.63 and 0.58 nM for rCsGSTo1 and 2). Interestingly, rCsGSTos exhibited high enzyme activity toward mu- and theta-class GST specific substrate, 4-nitrobenzyl chloride. Expression of CsGSTo transcripts and proteins increased beginning in 2-week-old juveniles and reached their highest levels in 4-week-old adults. The proteins were mainly expressed in the elements of the reproductive system, such as vitelline follicles, testes, seminal receptacle, sperm and eggs. Oxidative stressors induced upregulated expression of CsGSTos in these organs. Regardless of oxidative stresses, CsGSTos continued to be highly expressed in eggs. CsGSTo1 or 2 overexpressing bacteria demonstrated high resistance under oxidative killing.
Conclusions: CsGSTos might be critically involved in protection of the reproductive system during maturation of C. sinensis worms and in response to oxidative conditions, thereby contributing to maintenance of parasite fecundity.
Keywords: Clonorchis sinensis; Glutathione transferase (GST); Omega-class GST; Oxidative stress; Reproductive system; Sexual maturation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

