An autoradiographic evaluation of AV-1451 Tau PET in dementia
- PMID: 27296779
- PMCID: PMC4906968
- DOI: 10.1186/s40478-016-0315-6
An autoradiographic evaluation of AV-1451 Tau PET in dementia
Abstract
Background: It is essential to determine the specificity of AV-1451 PET for tau in brain imaging by using pathological comparisons. We performed autoradiography in autopsy-confirmed Alzheimer disease and other neurodegenerative disorders to evaluate the specificity of AV-1451 binding for tau aggregates.
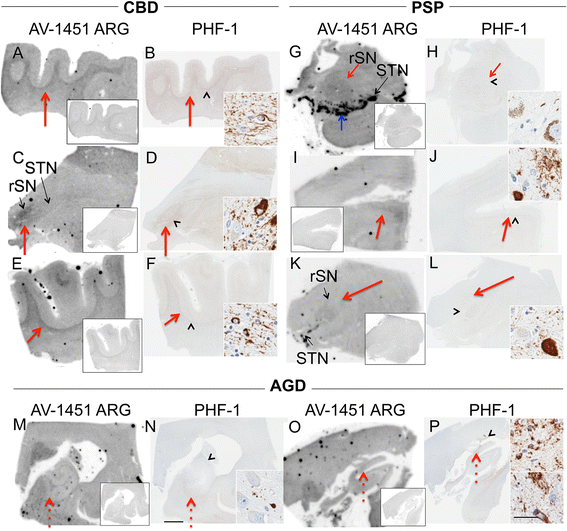
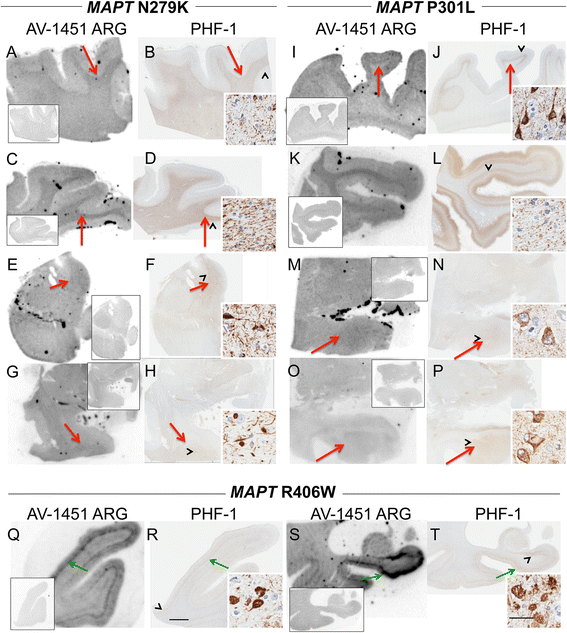
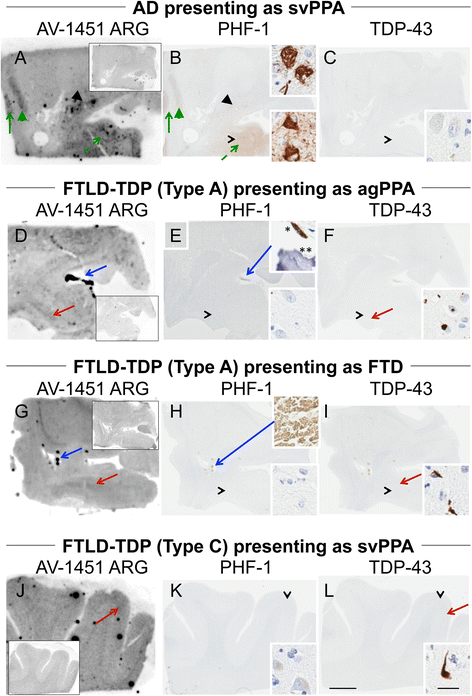
Methods: Tissue samples were selected that had a variety of dementia-related neuropathologies including Alzheimer disease, primary age-related tauopathy, tangle predominant dementia, non-Alzheimer disease tauopathies, frontotemporal dementia, parkinsonism, Lewy body disease and multiple system atrophy (n = 38). Brain tissue sections were stained for tau, TAR DNA-binding protein-43, and α-synuclein and compared to AV-1451 autoradiography on adjacent sections.
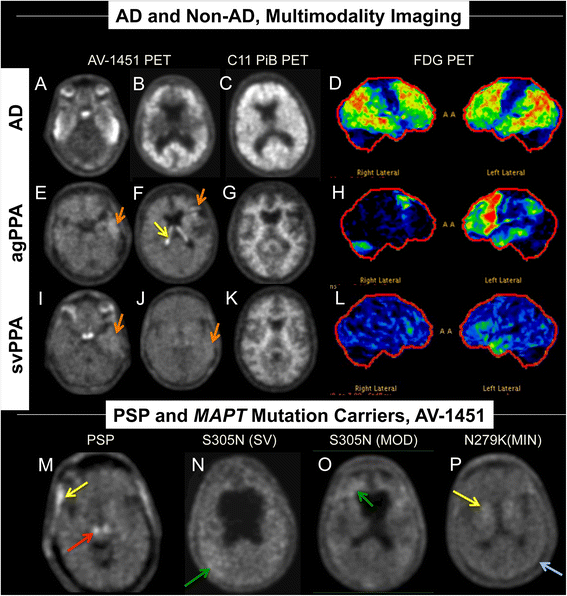
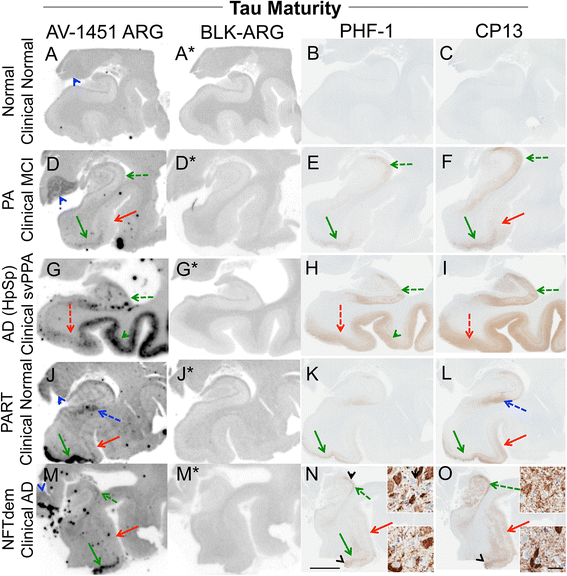
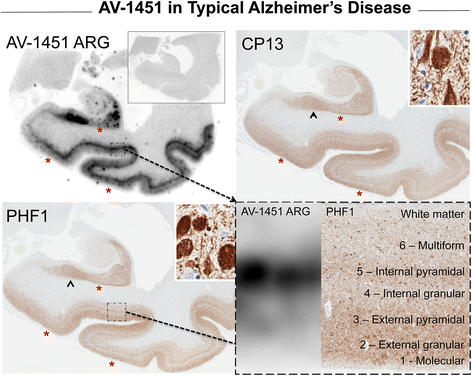
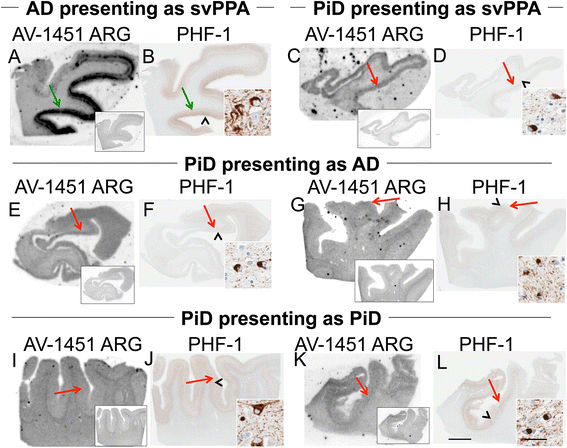
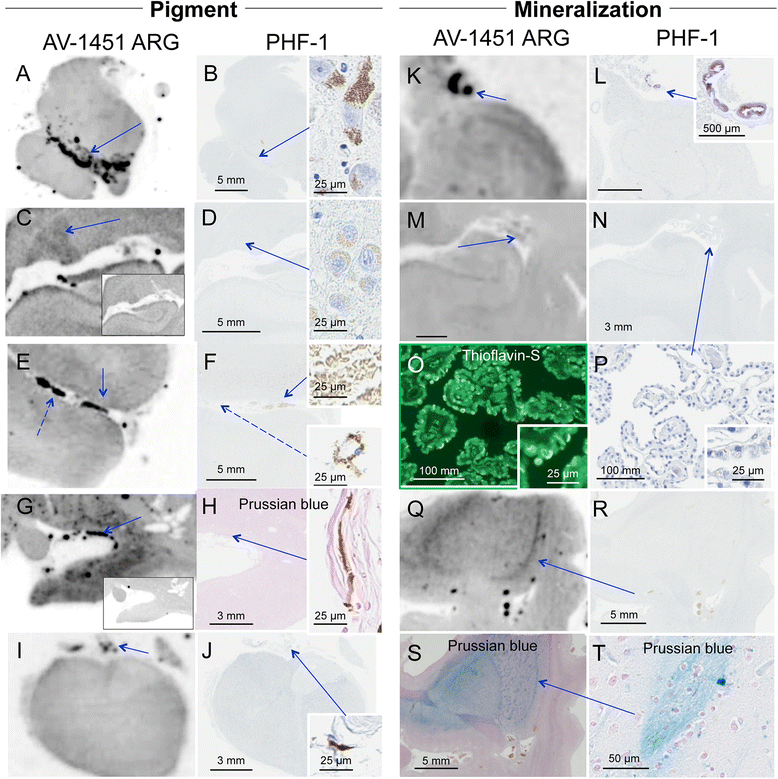
Results: AV-1451 preferentially localized to neurofibrillary tangles, with less binding to areas enriched in neuritic pathology and less mature tau. The strength of AV-1451 binding with respect to tau isoforms in various neurodegenerative disorders was: 3R + 4R tau (e.g., AD) > 3R tau (e.g., Pick disease) or 4R tau. Only minimal binding of AV-1451 to TAR DNA-binding protein-43 positive regions was detected. No binding of AV-1451 to α-synuclein was detected. "Off-target" binding was seen in vessels, iron-associated regions, substantia nigra, calcifications in the choroid plexus, and leptomeningeal melanin.
Conclusions: Reduced AV-1451 binding in neuritic pathology compared to neurofibrillary tangles suggests that the maturity of tau pathology may affect AV-1451 binding and suggests complexity in AV-1451 binding. Poor association of AV-1451 with tauopathies that have preferential accumulation of either 4R tau or 3R tau suggests limited clinical utility in detecting these pathologies. In contrast, for disorders associated with 3R + 4R tau, such as Alzheimer disease, AV-1451 binds tau avidly but does not completely reflect the early stage tau progression suggested by Braak neurofibrillary tangle staging. AV-1451 binding to TAR DNA-binding protein-43 or TAR DNA-binding protein-43 positive regions can be weakly positive. Clinical use of AV-1451 will require a familiarity with distinct types of "off-target" binding.
Keywords: AV-1451; Alzheimer’s disease; Atypical Alzheimer’s disease; Corticobasal degeneration; Frontotemporal dementia; Pick Disease; Pick’s disease; Progressive supranuclear palsy; TDP-43; Tau; Tauopathy.
Figures

References
-
- Janocko NJ, Brodersen KA, Soto-Ortolaza AI, Ross OA, Liesinger AM, Duara R, Graff-Radford NR, Dickson DW, Murray ME. Neuropathologically defined subtypes of Alzheimer’s disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol. 2012;124:681–692. doi: 10.1007/s00401-012-1044-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

