16S-ARDRA and MALDI-TOF mass spectrometry as tools for identification of Lactobacillus bacteria isolated from poultry
- PMID: 27296852
- PMCID: PMC4906599
- DOI: 10.1186/s12866-016-0732-5
16S-ARDRA and MALDI-TOF mass spectrometry as tools for identification of Lactobacillus bacteria isolated from poultry
Abstract
Background: The objective of our study is to evaluate the potential use of Amplified 16S Ribosomal DNA Restriction Analysis (16S-ARDRA) and MALDI-TOF mass spectrometry (MS) as methods for species identification of Lactobacillus strains in poultry.
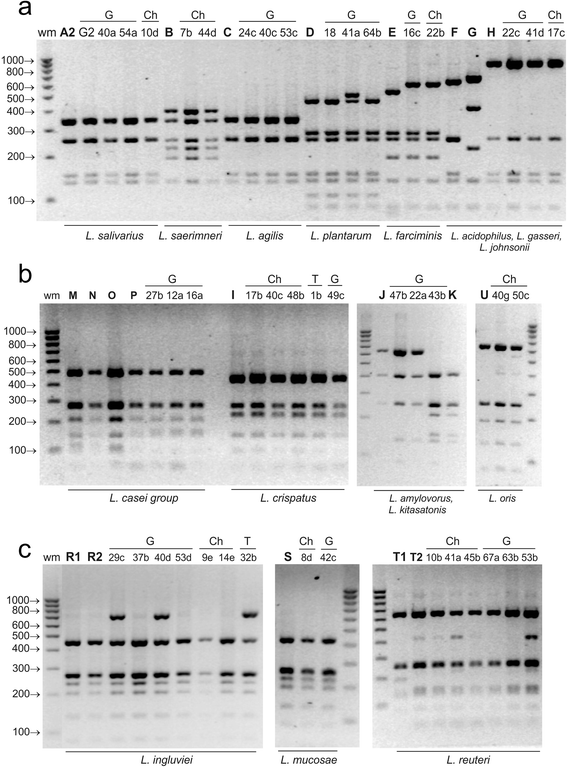
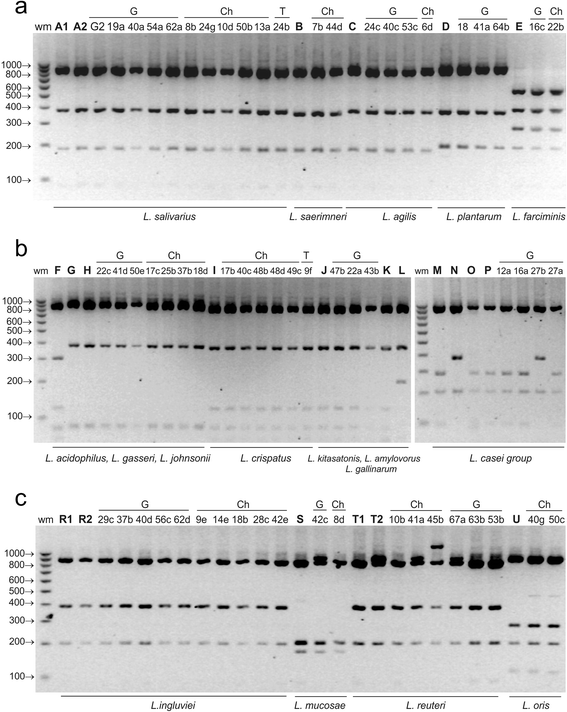
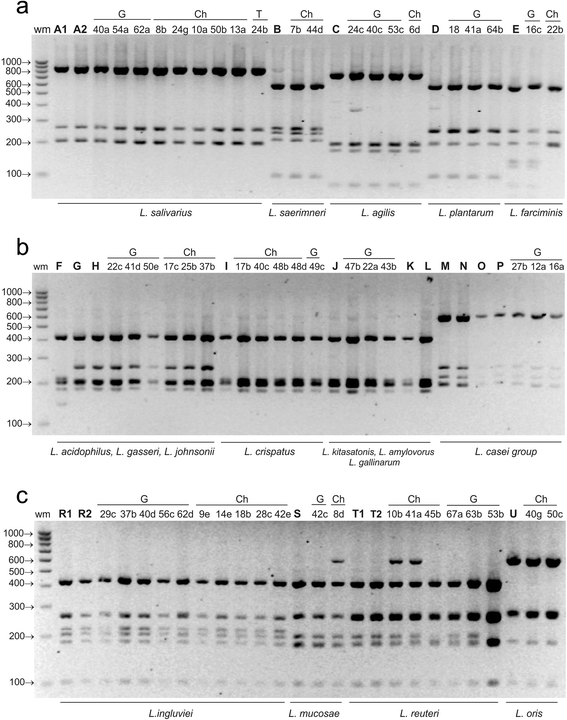
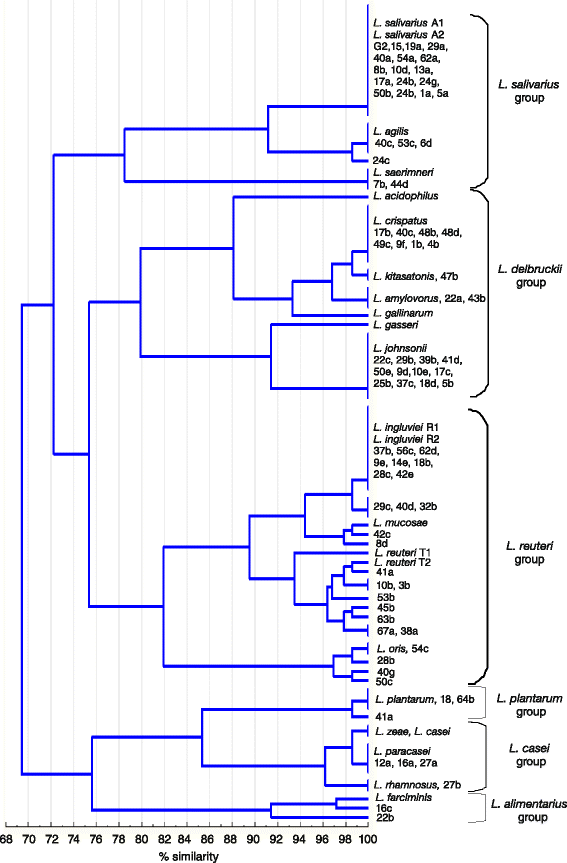
Results: A total of 80 Lactobacillus strains isolated from the cloaca of chicken, geese and turkeys were identified to the species level by MALDI-TOF MS (on-plate extraction method) and 16S-ARDRA. The two techniques produced comparable classification results, some of which were additionally confirmed by sequencing of 16S rDNA. MALDI-TOF MS enabled rapid species identification but produced more than one reliable identification result for 16.25 % of examined strains (mainly of the species L. johnsonii). For 30 % of isolates intermediate log(scores) of 1.70-1.99 were obtained, indicating correct genus identification but only presumptive species identification. The 16S-ARDRA protocol was based on digestion of 16S rDNA with the restriction enzymes MseI, HinfI, MboI and AluI. This technique was able to distinguish 17 of the 19 Lactobacillus reference species tested and enabled identification of all 80 wild isolates. L. salivarius dominated among the 15 recognized species, followed by L. johnsonii and L. ingluviei.
Conclusions: The MALDI-TOF MS and 16S-ARDRA assays are valuable tools for the identification of avian lactobacilli to the species level. MALDI-TOF MS is a fast, simple and cost-effective technique, and despite generating a high percentage of results with a log(score) <2.00, the on-plate extraction method is characterized by high-performance. For samples for which Biotyper produces more than one reliable result, MALDI-TOF MS must be used in combination with genotypic techniques to achieve unambiguous results. 16S-ARDRA is simple, repetitive method with high power of discrimination, whose sole limitation is its inability to discriminate between species with very high 16S rDNA sequence homology, such as L. casei and L. zeae. The assays can be used for discrimination of Lactobacillus bacteria from different habitats.
Keywords: 16S rDNA; ARDRA; Identification; Lactic acid bacteria; Lactobacillus; MALDI-TOF MS; Poultry.
Figures

References
-
- NCBI database. www.ncbi.nlm.nih.gov.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

