Molecularly Targeted Drugs Plus Radiotherapy and Temozolomide Treatment for Newly Diagnosed Glioblastoma: A Meta-Analysis and Systematic Review
- PMID: 27296952
- PMCID: PMC7838606
- DOI: 10.3727/096504016X14612603423511
Molecularly Targeted Drugs Plus Radiotherapy and Temozolomide Treatment for Newly Diagnosed Glioblastoma: A Meta-Analysis and Systematic Review
Abstract
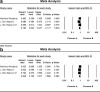
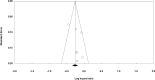
Glioblastoma (GBM) is the most common primary malignant brain tumor that nearly always results in a bad prognosis. Temozolomide plus radiotherapy (TEM+RAD) is the most common treatment for newly diagnosed GBM. With the development of molecularly targeted drugs, several clinical trials were reported; however, the efficacy of the treatment remains controversial. So we attempted to measure the dose of the molecularly targeted drug that could improve the prognosis of those patients. The appropriate electronic databases (PubMed, MEDLINE, EMBASE, and the Cochrane Library) were searched for relevant studies. A meta-analysis was performed after determining which studies met the inclusion criteria. Six randomized, controlled trials (RCTs) were identified for this meta-analysis, comprising 2,637 GBM patients. The benefit of overall survival (OS) was hazard ratio (HZ), 0.936 [95% confidence interval (CI), 0.852-1.028]. The benefit with respect to progression-free survival (PFS) rate was HZ of 0.796 (95% CI, 0.701-0.903). OS benefit of cilengitide was HZ of 0.792 (95% CI, 0.642-0.977). The adverse effects higher than grade 3 were 57.7% in the experimental group and 44.1% in the placebo group (odds ratio, 1.679; 95% CI, 1.434-1.967). The addition of molecularly targeted drugs to TEM + RAD did not improve the OS of patients with GBM; however, it did improve PFS in patients treated by cilengitide who could not get improvement in OS. The rate of adverse effects was higher in the experimental group than in the placebo group.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Kawano H.; Hirano H.; Yonezawa H.; Yunoue S.; Yatsushiro K.; Ogita M.; Hiraki Y.; Uchida H.; Habu M.; Fujio S.; Oyoshi T.; Bakhtiar Y.; Sugata S.; Yamahata H.; Hanaya R.; Tokimura H.; Arita K. Improvement in treatment results of glioblastoma over the last three decades and beneficial factors. Br. J. Neurosurg. 29:206–212; 2015. - PubMed
-
- Pope W. B.; Lai A.; Mehta R.; Kim H. J.; Qiao J.; Young J. R.; Xue X.; Goldin J.; Brown M. S.; Nghiemphu P. L.; Tran A.; Cloughesy T. F. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. Am. J. Neuroradiol. 32:882–889; 2011. - PMC - PubMed
-
- Chamberlain M. C. Treatment of newly diagnosed malignant glioma in the elderly people: New trials that impact therapy. Int. J. Clin. Pract. 67:1225–1227; 2013. - PubMed
-
- Nanegrungsunk D.; Onchan W.; Chattipakorn N.; Chattipakorn S. C. Current evidence of temozolomide and bevacizumab in treatment of gliomas. Neurol. Res. 37:167–183; 2015. - PubMed
-
- Rizzo D.; Scalzone M.; Ruggiero A.; Maurizi P.; Attina G.; Mastrangelo S.; Lazzareschi I.; Ridola V.; Colosimo C.; Caldarelli M.; Balducci M.; Riccardi R. Temozolomide in the treatment of newly diagnosed diffuse brainstem glioma in children: A broken promise? J. Chemother. 27:106–110; 2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
