Detergent Isolation Stabilizes and Activates the Shigella Type III Secretion System Translocator Protein IpaC
- PMID: 27297397
- PMCID: PMC4921279
- DOI: 10.1016/j.xphs.2016.05.015
Detergent Isolation Stabilizes and Activates the Shigella Type III Secretion System Translocator Protein IpaC
Abstract
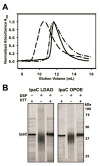
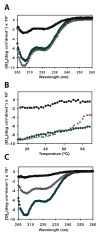
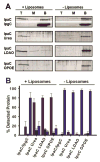
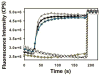
Shigella rely on a type III secretion system as the primary virulence factor for invasion and colonization of human hosts. Although there are an estimated 90 million Shigella infections, annually responsible for more than 100,000 deaths worldwide, challenges isolating and stabilizing many type III secretion system proteins have prevented a full understanding of the Shigella invasion mechanism and additionally slowed progress toward a much needed Shigella vaccine. Here, we show that the non-denaturing zwitterionic detergent N, N-dimethyldodecylamine N-oxide (LDAO) and non-ionic detergent n-octyl-oligo-oxyethylene efficiently isolated the hydrophobic Shigella translocator protein IpaC from the co-purified IpaC/IpgC chaperone-bound complex. Both detergents resulted in monomeric IpaC that exhibits strong membrane binding and lysis characteristics while the chaperone-bound complex does not, suggesting that the stabilizing detergents provide a means of following IpaC "activation" in vitro. Additionally, biophysical characterization found that LDAO provides significant thermal and temporal stability to IpaC, protecting it for several days at room temperature and brief exposure to temperatures reaching 90°C. In summary, this work identified and characterized conditions that provide stable, membrane active IpaC, providing insight into key interactions with membranes and laying a strong foundation for future vaccine formulation studies taking advantage of the native immunogenicity of IpaC and the stability provided by LDAO.
Keywords: circular dichroism; light scattering (dynamic); liposomes; physical characterization; physical stability.
Copyright © 2016 American Pharmacists Association®. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no personal financial or non-financial conflicts of interest.
Figures


References
-
- WHO. Initiative for Vaccine Research (IVR) Diarrhoeal Diseases. 2009 http://www.who.int/vaccine_research/diseases?diarrhoeal/en/index6.html.
-
- DuPont HL, Levine MM, Hornick RB, Formal SB. Inoculum size in shigellosis and implications for expected mode of transmission. The Journal of infectious diseases. 1989;159:1126–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

