New Insight into Daptomycin Bioavailability and Localization in Staphylococcus aureus Biofilms by Dynamic Fluorescence Imaging
- PMID: 27297479
- PMCID: PMC4958197
- DOI: 10.1128/AAC.00735-16
New Insight into Daptomycin Bioavailability and Localization in Staphylococcus aureus Biofilms by Dynamic Fluorescence Imaging
Abstract
Staphylococcus aureus is one of the most frequent pathogens responsible for biofilm-associated infections (BAI), and the choice of antibiotics to treat these infections remains a challenge for the medical community. In particular, daptomycin has been reported to fail against implant-associated S. aureus infections in clinical practice, while its association with rifampin remains a good candidate for BAI treatment. To improve our understanding of such resistance/tolerance toward daptomycin, we took advantage of the dynamic fluorescence imaging tools (time-lapse imaging and fluorescence recovery after photobleaching [FRAP]) to locally and accurately assess the antibiotic diffusion reaction in methicillin-susceptible and methicillin-resistant S. aureus biofilms. To provide a realistic representation of daptomycin action, we optimized an in vitro model built on the basis of our recently published in vivo mouse model of prosthetic vascular graft infections. We demonstrated that at therapeutic concentrations, daptomycin was inefficient in eradicating biofilms, while the matrix was not a shield to antibiotic diffusion and to its interaction with its bacterial target. In the presence of rifampin, daptomycin was still present in the vicinity of the bacterial cells, allowing prevention of the emergence of rifampin-resistant mutants. Conclusions derived from this study strongly suggest that S. aureus biofilm resistance/tolerance toward daptomycin may be more likely to be related to a physiological change involving structural modifications of the membrane, which is a strain-dependent process.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
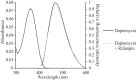
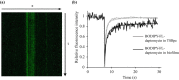
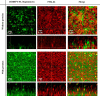


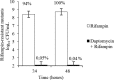
References
-
- Lebeaux D, Ghigo J-M, Beloin C. 2014. Tolérance des biofilms aux antibiotiques: comprendre pour mieux traiter. J Anti-Infect 16:112–121. doi:10.1016/j.antinf.2014.04.001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

