GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism
- PMID: 27297692
- PMCID: PMC4906353
- DOI: 10.1038/srep27698
GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism
Abstract
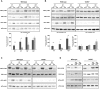
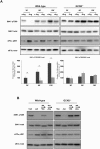
It is well known that the GCN2 and mTORC1 signaling pathways are regulated by amino acids and share common functions, in particular the control of translation. The regulation of GCN2 activity by amino acid availability relies on the capacity of GCN2 to sense the increased levels of uncharged tRNAs upon amino acid scarcity. In contrast, despite recent progress in the understanding of the regulation of mTORC1 by amino acids, key aspects of this process remain unsolved. In particular, while leucine is well known to be a potent regulator of mTORC1, the mechanisms by which this amino acid is sensed and control mTORC1 activity are not well defined. Our data establish that GCN2 is involved in the inhibition of mTORC1 upon leucine or arginine deprivation. However, the activation of GCN2 alone is not sufficient to inhibit mTORC1 activity, indicating that leucine and arginine exert regulation via additional mechanisms. While the mechanism by which GCN2 contributes to the initial step of mTORC1 inhibition involves the phosphorylation of eIF2α, we show that it is independent of the downstream transcription factor ATF4. These data point to a novel role for GCN2 and phosphorylation of eIF2α in the control of mTORC1 by certain amino acids.
Figures



References
-
- Kim D.-H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002). - PubMed
-
- Hara K. et al. Amino Acid Sufficiency and mTOR Regulate p70 S6 Kinase and eIF-4E BP1 through a Common Effector Mechanism. J. Biol. Chem. 273, 14484–14494 (1998). - PubMed
-
- Berlanga J. J., Santoyo J. & de Haro C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur. J. Biochem. 265, 754–762 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

