Highly regio- and enantioselective multiple oxy- and amino-functionalizations of alkenes by modular cascade biocatalysis
- PMID: 27297777
- PMCID: PMC4911676
- DOI: 10.1038/ncomms11917
Highly regio- and enantioselective multiple oxy- and amino-functionalizations of alkenes by modular cascade biocatalysis
Abstract
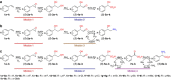
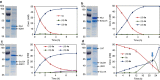
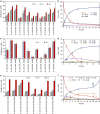
New types of asymmetric functionalizations of alkenes are highly desirable for chemical synthesis. Here, we develop three novel types of regio- and enantioselective multiple oxy- and amino-functionalizations of terminal alkenes via cascade biocatalysis to produce chiral α-hydroxy acids, 1,2-amino alcohols and α-amino acids, respectively. Basic enzyme modules 1-4 are developed to convert alkenes to (S)-1,2-diols, (S)-1,2-diols to (S)-α-hydroxyacids, (S)-1,2-diols to (S)-aminoalcohols and (S)-α-hydroxyacids to (S)-α-aminoacids, respectively. Engineering of enzyme modules 1 &2, 1 &3 and 1, 2 &4 in Escherichia coli affords three biocatalysts over-expressing 4-8 enzymes for one-pot conversion of styrenes to the corresponding (S)-α-hydroxyacids, (S)-aminoalcohols and (S)-α-aminoacids in high e.e. and high yields, respectively. The new types of asymmetric alkene functionalizations provide green, safe and useful alternatives to the chemical syntheses of these compounds. The modular approach for engineering multi-step cascade biocatalysis is useful for developing other new types of one-pot biotransformations for chemical synthesis.
Conflict of interest statement
Z.L. and S.W. are the co-inventors on two patent applications: ‘Production of enantiopure α-hydroxy carboxylic acids from alkenes by cascade biocatalysis' PCT application number PCT/SG2014/000221; and ‘Production of chiral 1,2-amino alcohols and α-amino acids from alkenes by cascade biocatalysis' US provisional application 62/283,508.
Figures

Similar articles
-
Enantioselective Aminohydroxylation of Styrenyl Olefins Catalyzed by an Engineered Hemoprotein.Angew Chem Int Ed Engl. 2019 Mar 4;58(10):3138-3142. doi: 10.1002/anie.201812968. Epub 2019 Jan 25. Angew Chem Int Ed Engl. 2019. PMID: 30600873
-
One pot simultaneous preparation of both enantiomer of β-amino alcohol and vicinal diol via cascade biocatalysis.Biotechnol Lett. 2018 Feb;40(2):349-358. doi: 10.1007/s10529-017-2471-6. Epub 2017 Nov 9. Biotechnol Lett. 2018. PMID: 29124518
-
Amino acid-based reoxidants for aminohydroxylation: application to the construction of amino acid-amino alcohol conjugates.Angew Chem Int Ed Engl. 2011 Nov 11;50(46):10957-60. doi: 10.1002/anie.201103293. Epub 2011 Sep 23. Angew Chem Int Ed Engl. 2011. PMID: 21953892 No abstract available.
-
Green and Enantioselective Synthesis via Cascade Biotransformations: From Simple Racemic Substrates to High-Value Chiral Chemicals.Chem Asian J. 2024 Oct 1;19(19):e202400565. doi: 10.1002/asia.202400565. Epub 2024 Aug 21. Chem Asian J. 2024. PMID: 38954385 Review.
-
Fluorescence of organic molecules in chiral recognition.Chem Rev. 2004 Mar;104(3):1687-716. doi: 10.1021/cr030052h. Chem Rev. 2004. PMID: 15008630 Review. No abstract available.
Cited by
-
Ligand Controlled Ir-Catalyzed Regiodivergent Oxyamination of Unactivated Alkenes.J Am Chem Soc. 2019 Jul 31;141(30):11864-11869. doi: 10.1021/jacs.9b06366. Epub 2019 Jul 16. J Am Chem Soc. 2019. PMID: 31310537 Free PMC article.
-
Metabolic engineering of Pseudomonas putida for production of vanillylamine from lignin-derived substrates.Microb Biotechnol. 2021 Nov;14(6):2448-2462. doi: 10.1111/1751-7915.13764. Epub 2021 Feb 3. Microb Biotechnol. 2021. PMID: 33533574 Free PMC article.
-
Efficient synthesis of enantiopure amines from alcohols using resting E. coli cells and ammonia.Green Chem. 2019 Jul 14;21(14):3846-3857. doi: 10.1039/C9GC01059A. Epub 2019 Jun 25. Green Chem. 2019. PMID: 33628111 Free PMC article.
-
Two-Component FAD-Dependent Monooxygenases: Current Knowledge and Biotechnological Opportunities.Biology (Basel). 2018 Aug 2;7(3):42. doi: 10.3390/biology7030042. Biology (Basel). 2018. PMID: 30072664 Free PMC article. Review.
-
Production of Aldehydes by Biocatalysis.Int J Mol Sci. 2021 May 6;22(9):4949. doi: 10.3390/ijms22094949. Int J Mol Sci. 2021. PMID: 34066641 Free PMC article. Review.
References
-
- Katsuki T. & Sharpless K. B. The first practical method for asymmetric epoxidation. J. Am. Chem. Soc. 102, 5974–5976 (1980).
-
- Kolb H. C., VanNieuwenhze M. S. & Sharpless K. B. Catalytic asymmetric dihydroxylation. Chem. Rev. 94, 2483–2547 (1994).
-
- Li G., Chang H. T. & Sharpless K. B. Catalytic asymmetric aminohydroxylation (AA) of olefins. Angew. Chem. Int. Ed. 35, 451–454 (1996).
-
- Zhang W., Loebach J. L., Wilson S. R. & Jacobsen E. N. Enantioselective epoxidation of unfunctionalized olefins catalysed by salen manganese complexes. J. Am. Chem. Soc. 112, 2801–2803 (1990).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

