Highly regio- and enantioselective multiple oxy- and amino-functionalizations of alkenes by modular cascade biocatalysis
- PMID: 27297777
- PMCID: PMC4911676
- DOI: 10.1038/ncomms11917
Highly regio- and enantioselective multiple oxy- and amino-functionalizations of alkenes by modular cascade biocatalysis
Abstract
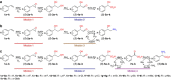
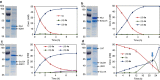
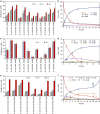
New types of asymmetric functionalizations of alkenes are highly desirable for chemical synthesis. Here, we develop three novel types of regio- and enantioselective multiple oxy- and amino-functionalizations of terminal alkenes via cascade biocatalysis to produce chiral α-hydroxy acids, 1,2-amino alcohols and α-amino acids, respectively. Basic enzyme modules 1-4 are developed to convert alkenes to (S)-1,2-diols, (S)-1,2-diols to (S)-α-hydroxyacids, (S)-1,2-diols to (S)-aminoalcohols and (S)-α-hydroxyacids to (S)-α-aminoacids, respectively. Engineering of enzyme modules 1 &2, 1 &3 and 1, 2 &4 in Escherichia coli affords three biocatalysts over-expressing 4-8 enzymes for one-pot conversion of styrenes to the corresponding (S)-α-hydroxyacids, (S)-aminoalcohols and (S)-α-aminoacids in high e.e. and high yields, respectively. The new types of asymmetric alkene functionalizations provide green, safe and useful alternatives to the chemical syntheses of these compounds. The modular approach for engineering multi-step cascade biocatalysis is useful for developing other new types of one-pot biotransformations for chemical synthesis.
Conflict of interest statement
Z.L. and S.W. are the co-inventors on two patent applications: ‘Production of enantiopure α-hydroxy carboxylic acids from alkenes by cascade biocatalysis' PCT application number PCT/SG2014/000221; and ‘Production of chiral 1,2-amino alcohols and α-amino acids from alkenes by cascade biocatalysis' US provisional application 62/283,508.
Figures

References
-
- Katsuki T. & Sharpless K. B. The first practical method for asymmetric epoxidation. J. Am. Chem. Soc. 102, 5974–5976 (1980).
-
- Kolb H. C., VanNieuwenhze M. S. & Sharpless K. B. Catalytic asymmetric dihydroxylation. Chem. Rev. 94, 2483–2547 (1994).
-
- Li G., Chang H. T. & Sharpless K. B. Catalytic asymmetric aminohydroxylation (AA) of olefins. Angew. Chem. Int. Ed. 35, 451–454 (1996).
-
- Zhang W., Loebach J. L., Wilson S. R. & Jacobsen E. N. Enantioselective epoxidation of unfunctionalized olefins catalysed by salen manganese complexes. J. Am. Chem. Soc. 112, 2801–2803 (1990).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

