High-content screening identifies kinase inhibitors that overcome venetoclax resistance in activated CLL cells
- PMID: 27297795
- PMCID: PMC5000578
- DOI: 10.1182/blood-2015-12-687814
High-content screening identifies kinase inhibitors that overcome venetoclax resistance in activated CLL cells
Abstract
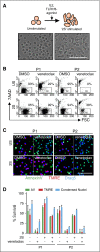
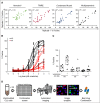
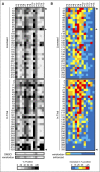
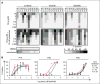
Novel agents such as the Bcl-2 inhibitor venetoclax (ABT-199) are changing treatment paradigms for chronic lymphocytic leukemia (CLL) but important problems remain. Although some patients exhibit deep and durable responses to venetoclax as a single agent, other patients harbor subpopulations of resistant leukemia cells that mediate disease recurrence. One hypothesis for the origin of resistance to venetoclax is by kinase-mediated survival signals encountered in proliferation centers that may be unique for individual patients. An in vitro microenvironment model was developed with primary CLL cells that could be incorporated into an automated high-content microscopy-based screen of kinase inhibitors (KIs) to identify agents that may improve venetoclax therapy in a personalized manner. Marked interpatient variability was noted for which KIs were effective; nevertheless, sunitinib was identified as the most common clinically available KI effective in overcoming venetoclax resistance. Examination of the underlying mechanisms indicated that venetoclax resistance may be induced by microenvironmental signals that upregulate antiapoptotic Bcl-xl, Mcl-1, and A1, which can be counteracted more efficiently by sunitinib than by ibrutinib or idelalisib. Although patient-specific drug responses are common, for many patients, combination therapy with sunitinib may significantly improve the therapeutic efficacy of venetoclax.
© 2016 by The American Society of Hematology.
Figures


References
-
- Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14(9):2519–2526. - PubMed
-
- Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–2663. - PubMed
-
- Vogler M, Butterworth M, Majid A, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–4413. - PubMed
-
- Brahmbhatt H, Oppermann S, Osterlund EJ, Leber B, Andrews DW. Molecular pathways: leveraging the BCL-2 interactome to kill cancer cells--mitochondrial outer membrane permeabilization and beyond. Clin Cancer Res. 2015;21(12):2671–2676. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

