Impact of peanut consumption in the LEAP Study: Feasibility, growth, and nutrition
- PMID: 27297994
- PMCID: PMC5056823
- DOI: 10.1016/j.jaci.2016.04.016
Impact of peanut consumption in the LEAP Study: Feasibility, growth, and nutrition
Abstract
Background: Early introduction of peanut is an effective strategy to prevent peanut allergy in high-risk infants; however, feasibility and effects on growth and nutritional intake are unknown.
Objective: We sought to evaluate the feasibility of introducing peanut in infancy and explore effects on growth and nutritional intake up to age 60 months.
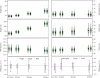
Methods: In the Learning Early About Peanut Allergy trial, 640 atopic infants aged 4 to 11 months were randomly assigned to consume (6 g peanut protein per week) or avoid peanut until age 60 months. Peanut consumption and early feeding practices were assessed by questionnaire. Dietary intake was evaluated with prospective food diaries. Anthropometric measurements were taken at all study visits.
Results: Peanut was successfully introduced and consumed until 60 months, with median peanut protein intake of 7.5 g/wk (interquartile range, 6.0-9.0 g/wk) in the consumption group compared with 0 g in the avoidance group. Introduction of peanut in breast-feeding infants did not affect the duration of breast-feeding. There were no differences in anthropometric measurements or energy intakes between groups at any visits. Regular peanut consumption led to differences in dietary intakes. Consumers had higher intakes of fat and avoiders had higher carbohydrate intakes; differences were greatest at the upper quartiles of peanut consumption. Protein intakes remained consistent between groups.
Conclusions: Introduction of peanut proved feasible in infants at high risk of peanut allergy and did not affect the duration of breast-feeding nor impact negatively on growth or nutrition. Energy balance was achieved in both groups through variations in intakes from fat and carbohydrate while protein homeostasis was maintained.
Keywords: Food allergy; allergy prevention; breast-feeding; growth; infant feeding; nutrition; peanut; prospective food diary; protein homeostasis.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Figures


Comment in
-
LEAPing ahead with early allergen consumption.J Allergy Clin Immunol. 2016 Oct;138(4):1119-1121. doi: 10.1016/j.jaci.2016.08.001. Epub 2016 Aug 24. J Allergy Clin Immunol. 2016. PMID: 27566457 No abstract available.
-
Reply.J Allergy Clin Immunol. 2017 Apr;139(4):1407. doi: 10.1016/j.jaci.2016.11.025. Epub 2017 Jan 27. J Allergy Clin Immunol. 2017. PMID: 28139315 No abstract available.
-
Early or delayed introduction of food? Misunderstanding is in the air.J Allergy Clin Immunol. 2017 Apr;139(4):1405-1406. doi: 10.1016/j.jaci.2016.11.026. Epub 2017 Jan 27. J Allergy Clin Immunol. 2017. PMID: 28139317 No abstract available.
References
-
- World Health Organization (WHO) Fifty-fifth World Health Assembly Infant and young child nutition. Global strategy on infant and young child feeding. 2002;A55/15
-
- Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008 Nov;122(5):984–991. - PubMed
-
- Hourihane JO, Aiken R, Briggs R, Gudgeon LA, Grimshaw KEC, DunnGalvin A, et al. The impact of government advice to pregnant mothers regarding peanut avoidance on the prevalence of peanut allergy in United Kingdom children at school entry. J Allergy Clin Immunol. 2007;119(5):1197–1202. 5. - PubMed
-
- Health and Social Care Information Centre (HSCIC) Infant Feeding Survey. 2010;2012
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

