Ligand Binding Ensembles Determine Graded Agonist Efficacies at a G Protein-coupled Receptor
- PMID: 27298318
- PMCID: PMC4965584
- DOI: 10.1074/jbc.M116.735431
Ligand Binding Ensembles Determine Graded Agonist Efficacies at a G Protein-coupled Receptor
Abstract
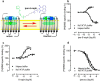
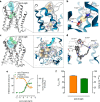
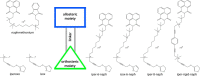
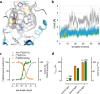
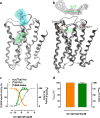
G protein-coupled receptors constitute the largest family of membrane receptors and modulate almost every physiological process in humans. Binding of agonists to G protein-coupled receptors induces a shift from inactive to active receptor conformations. Biophysical studies of the dynamic equilibrium of receptors suggest that a portion of receptors can remain in inactive states even in the presence of saturating concentrations of agonist and G protein mimetic. However, the molecular details of agonist-bound inactive receptors are poorly understood. Here we use the model of bitopic orthosteric/allosteric (i.e. dualsteric) agonists for muscarinic M2 receptors to demonstrate the existence and function of such inactive agonist·receptor complexes on a molecular level. Using all-atom molecular dynamics simulations, dynophores (i.e. a combination of static three-dimensional pharmacophores and molecular dynamics-based conformational sampling), ligand design, and receptor mutagenesis, we show that inactive agonist·receptor complexes can result from agonist binding to the allosteric vestibule alone, whereas the dualsteric binding mode produces active receptors. Each agonist forms a distinct ligand binding ensemble, and different agonist efficacies depend on the fraction of purely allosteric (i.e. inactive) versus dualsteric (i.e. active) binding modes. We propose that this concept may explain why agonist·receptor complexes can be inactive and that adopting multiple binding modes may be generalized also to small agonists where binding modes will be only subtly different and confined to only one binding site.
Keywords: G protein-coupled receptor (GPCR); chemical biology; drug design; dualsteric ligands; dynamic ligand binding; dynophores; ligand binding ensembles; molecular dynamics; molecular pharmacology; partial agonist.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
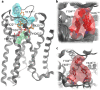


References
-
- Fredriksson R., Lagerström M. C., Lundin L.-G., and Schiöth H. B. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 - PubMed
-
- Venkatakrishnan A. J., Deupi X., Lebon G., Tate C. G., Schertler G. F., and Babu M. M. (2013) Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions

