Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling
- PMID: 27298345
- PMCID: PMC4932970
- DOI: 10.1073/pnas.1604519113
Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling
Erratum in
-
Correction for Yin et al., Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling.Proc Natl Acad Sci U S A. 2025 Jun 10;122(23):e2511044122. doi: 10.1073/pnas.2511044122. Epub 2025 May 30. Proc Natl Acad Sci U S A. 2025. PMID: 40445774 Free PMC article. No abstract available.
Abstract
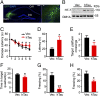
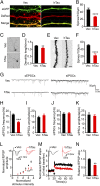
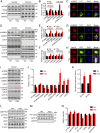
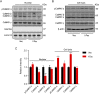
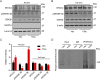
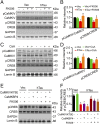
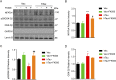
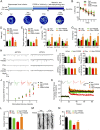
Intracellular accumulation of wild-type tau is a hallmark of sporadic Alzheimer's disease (AD), but the molecular mechanisms underlying tau-induced synapse impairment and memory deficit are poorly understood. Here we found that overexpression of human wild-type full-length tau (termed hTau) induced memory deficits with impairments of synaptic plasticity. Both in vivo and in vitro data demonstrated that hTau accumulation caused remarkable dephosphorylation of cAMP response element binding protein (CREB) in the nuclear fraction. Simultaneously, the calcium-dependent protein phosphatase calcineurin (CaN) was up-regulated, whereas the calcium/calmodulin-dependent protein kinase IV (CaMKIV) was suppressed. Further studies revealed that CaN activation could dephosphorylate CREB and CaMKIV, and the effect of CaN on CREB dephosphorylation was independent of CaMKIV inhibition. Finally, inhibition of CaN attenuated the hTau-induced CREB dephosphorylation with improved synapse and memory functions. Together, these data indicate that the hTau accumulation impairs synapse and memory by CaN-mediated suppression of nuclear CaMKIV/CREB signaling. Our findings not only reveal new mechanisms underlying the hTau-induced synaptic toxicity, but also provide potential targets for rescuing tauopathies.
Keywords: Alzheimer’s disease; CREB; Ca2+/calmodulin-dependent kinase IV; calcineurin; tau.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. - PubMed
-
- Glenner GG, Wong CW. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. - PubMed
-
- Grundke-Iqbal I, et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261(13):6084–6089. - PubMed
-
- Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J Neurol Sci. 1987;78(2):151–164. - PubMed
-
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol. 1990;27(5):457–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

