δ-COP contains a helix C-terminal to its longin domain key to COPI dynamics and function
- PMID: 27298352
- PMCID: PMC4922195
- DOI: 10.1073/pnas.1603544113
δ-COP contains a helix C-terminal to its longin domain key to COPI dynamics and function
Abstract
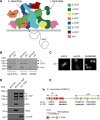
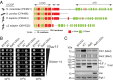
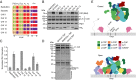
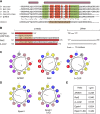
Membrane recruitment of coatomer and formation of coat protein I (COPI)-coated vesicles is crucial to homeostasis in the early secretory pathway. The conformational dynamics of COPI during cargo capture and vesicle formation is incompletely understood. By scanning the length of δ-COP via functional complementation in yeast, we dissect the domains of the δ-COP subunit. We show that the μ-homology domain is dispensable for COPI function in the early secretory pathway, whereas the N-terminal longin domain is essential. We map a previously uncharacterized helix, C-terminal to the longin domain, that is specifically required for the retrieval of HDEL-bearing endoplasmic reticulum-luminal residents. It is positionally analogous to an unstructured linker that becomes helical and membrane-facing in the open form of the AP2 clathrin adaptor complex. Based on the amphipathic nature of the critical helix it may probe the membrane for lipid packing defects or mediate interaction with cargo and thus contribute to stabilizing membrane-associated coatomer.
Keywords: ARCN1; COPI; HDEL; KDEL; coatomer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

