Closed state-coupled C-type inactivation in BK channels
- PMID: 27298368
- PMCID: PMC4922147
- DOI: 10.1073/pnas.1607584113
Closed state-coupled C-type inactivation in BK channels
Abstract
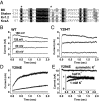
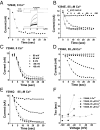
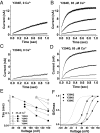
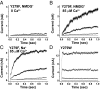
Ion channels regulate ion flow by opening and closing their pore gates. K(+) channels commonly possess two pore gates, one at the intracellular end for fast channel activation/deactivation and the other at the selectivity filter for slow C-type inactivation/recovery. The large-conductance calcium-activated potassium (BK) channel lacks a classic intracellular bundle-crossing activation gate and normally show no C-type inactivation. We hypothesized that the BK channel's activation gate may spatially overlap or coexist with the C-type inactivation gate at or near the selectivity filter. We induced C-type inactivation in BK channels and studied the relationship between activation/deactivation and C-type inactivation/recovery. We observed prominent slow C-type inactivation/recovery in BK channels by an extreme low concentration of extracellular K(+) together with a Y294E/K/Q/S or Y279F mutation whose equivalent in Shaker channels (T449E/K/D/Q/S or W434F) caused a greatly accelerated rate of C-type inactivation or constitutive C-inactivation. C-type inactivation in most K(+) channels occurs upon sustained membrane depolarization or channel opening and then recovers during hyperpolarized membrane potentials or channel closure. However, we found that the BK channel C-type inactivation occurred during hyperpolarized membrane potentials or with decreased intracellular calcium ([Ca(2+)]i) and recovered with depolarized membrane potentials or elevated [Ca(2+)]i Constitutively open mutation prevented BK channels from C-type inactivation. We concluded that BK channel C-type inactivation is closed state-dependent and that its extents and rates inversely correlate with channel-open probability. Because C-type inactivation can involve multiple conformational changes at the selectivity filter, we propose that the BK channel's normal closing may represent an early conformational stage of C-type inactivation.
Keywords: BK channel; C-type inactivation; maxi K channel; pore gate; potassium channel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
An S6 mutation in BK channels reveals beta1 subunit effects on intrinsic and voltage-dependent gating.J Gen Physiol. 2006 Dec;128(6):731-44. doi: 10.1085/jgp.200609596. J Gen Physiol. 2006. PMID: 17130522 Free PMC article.
-
Selectivity filter gating in large-conductance Ca(2+)-activated K+ channels.J Gen Physiol. 2012 Mar;139(3):235-44. doi: 10.1085/jgp.201110748. J Gen Physiol. 2012. PMID: 22371364 Free PMC article.
-
State-dependent block of BK channels by synthesized shaker ball peptides.J Gen Physiol. 2006 Oct;128(4):423-41. doi: 10.1085/jgp.200609521. Epub 2006 Sep 11. J Gen Physiol. 2006. PMID: 16966472 Free PMC article.
-
Molecular mechanisms of BK channel activation.Cell Mol Life Sci. 2009 Mar;66(5):852-75. doi: 10.1007/s00018-008-8609-x. Cell Mol Life Sci. 2009. PMID: 19099186 Free PMC article. Review.
-
Protein Network Interacting with BK Channels.Int Rev Neurobiol. 2016;128:127-61. doi: 10.1016/bs.irn.2016.03.003. Epub 2016 Mar 28. Int Rev Neurobiol. 2016. PMID: 27238263 Review.
Cited by
-
Eukaryotic Kv channel Shaker inactivates through selectivity filter dilation rather than collapse.Sci Adv. 2023 Dec 8;9(49):eadj5539. doi: 10.1126/sciadv.adj5539. Epub 2023 Dec 8. Sci Adv. 2023. PMID: 38064553 Free PMC article.
-
Structure of the Shaker Kv channel and mechanism of slow C-type inactivation.Sci Adv. 2022 Mar 18;8(11):eabm7814. doi: 10.1126/sciadv.abm7814. Epub 2022 Mar 18. Sci Adv. 2022. PMID: 35302848 Free PMC article.
-
Mutations within the selectivity filter reveal that Kv1 channels have distinct propensities to slow inactivate.J Gen Physiol. 2022 Nov 7;154(11):e202213222. doi: 10.1085/jgp.202213222. Epub 2022 Oct 5. J Gen Physiol. 2022. PMID: 36197416 Free PMC article.
-
Activation and closed-state inactivation mechanisms of the human voltage-gated KV4 channel complexes.Mol Cell. 2022 Jul 7;82(13):2427-2442.e4. doi: 10.1016/j.molcel.2022.04.032. Epub 2022 May 20. Mol Cell. 2022. PMID: 35597238 Free PMC article.
-
Transmembrane determinants of voltage-gating differences between BK (Slo1) and Slo3 channels.Biophys J. 2024 Jul 16;123(14):2154-2166. doi: 10.1016/j.bpj.2024.04.016. Epub 2024 Apr 18. Biophys J. 2024. PMID: 38637987 Free PMC article.
References
-
- Yellen G. The moving parts of voltage-gated ion channels. Q Rev Biophys. 1998;31(3):239–295. - PubMed
-
- del Camino D, Yellen G. Tight steric closure at the intracellular activation gate of a voltage-gated K(+) channel. Neuron. 2001;32(4):649–656. - PubMed
-
- Lü Q, Miller C. Silver as a probe of pore-forming residues in a potassium channel. Science. 1995;268(5208):304–307. - PubMed
-
- Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. - PubMed
-
- Kukuljan M, Labarca P, Latorre R. Molecular determinants of ion conduction and inactivation in K+ channels. Am J Physiol. 1995;268(3 Pt 1):C535–C556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

