Inhibition of the Aspergillus flavus Growth and Aflatoxin B1 Contamination on Pistachio Nut by Fengycin and Surfactin-Producing Bacillus subtilis UTBSP1
- PMID: 27298596
- PMCID: PMC4892817
- DOI: 10.5423/PPJ.OA.11.2015.0250
Inhibition of the Aspergillus flavus Growth and Aflatoxin B1 Contamination on Pistachio Nut by Fengycin and Surfactin-Producing Bacillus subtilis UTBSP1
Abstract
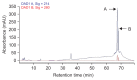
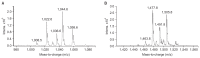
In this study, the treatment of pistachio nuts by Bacillus subtilis UTBSP1, a promising isolate to degrade aflatoxin B1 (AFB1), caused to reduce the growth of Aspergillus flavus R5 and AFB1 content on pistachio nuts. Fluorescence probes revealed that the cell free supernatant fluid from UTBSP1 affects spore viability considerably. Using high-performance liquid chromatographic (HPLC) method, 10 fractions were separated and collected from methanol extract of cell free supernatant fluid. Two fractions showed inhibition zones against A. flavus. Mass spectrometric analysis of the both antifungal fractions revealed a high similarity between these anti-A. flavus compounds and cyclic-lipopeptides of surfactin, and fengycin families. Coproduction of surfactin and fengycin acted in a synergistic manner and consequently caused a strong antifungal activity against A. flavus R5. There was a positive significant correlation between the reduction of A. flavus growth and the reduction of AFB1 contamination on pistachio nut by UTBSP1. The results indicated that fengycin and surfactin-producing B. subtilis UTBSP1 can potentially reduce A. flavus growth and AFB1 content in pistachio nut.
Keywords: Bacillus subtilis; aflatoxin B1; antifungal activity; fengycin; surfactin.
Figures



Similar articles
-
Interacting Abiotic Factors Affect Growth and Aflatoxin B1 Production Profiles of Aspergillus flavus Strains on Pistachio-Based Matrices and Pistachio Nuts.Front Microbiol. 2021 Jan 20;11:624007. doi: 10.3389/fmicb.2020.624007. eCollection 2020. Front Microbiol. 2021. PMID: 33552034 Free PMC article.
-
Impacts of Climate Change Interacting Abiotic Factors on Growth, aflD and aflR Gene Expression and Aflatoxin B1 Production by Aspergillus flavus Strains In Vitro and on Pistachio Nuts.Toxins (Basel). 2021 May 28;13(6):385. doi: 10.3390/toxins13060385. Toxins (Basel). 2021. PMID: 34071166 Free PMC article.
-
An attempt to model the probability of growth and aflatoxin B1 production of Aspergillus flavus under non-isothermal conditions in pistachio nuts.Food Microbiol. 2015 Oct;51:117-29. doi: 10.1016/j.fm.2015.05.013. Epub 2015 May 27. Food Microbiol. 2015. PMID: 26187836
-
Exogenous β-aminobutyric acid application attenuates Aspergillus decay, minimizes aflatoxin B1 accumulation, and maintains nutritional quality in fresh-in-hull pistachio kernels.J Sci Food Agric. 2020 Mar 30;100(5):2130-2135. doi: 10.1002/jsfa.10236. Epub 2020 Jan 14. J Sci Food Agric. 2020. PMID: 31884686
-
Molecular and biochemical characterization of Iranian surfactin-producing Bacillus subtilis isolates and evaluation of their biocontrol potential against Aspergillus flavus and Colletotrichum gloeosporioides.Can J Microbiol. 2009 Apr;55(4):395-404. doi: 10.1139/w08-141. Can J Microbiol. 2009. PMID: 19396239
Cited by
-
Bacillus megaterium WL-3 Lipopeptides Collaborate Against Phytophthora infestans to Control Potato Late Blight and Promote Potato Plant Growth.Front Microbiol. 2020 Jul 9;11:1602. doi: 10.3389/fmicb.2020.01602. eCollection 2020. Front Microbiol. 2020. PMID: 32733429 Free PMC article.
-
Biocontrol Capabilities of Bacillus subtilis E11 against Aspergillus flavus In Vitro and for Dried Red Chili (Capsicum annuum L.).Toxins (Basel). 2023 Apr 26;15(5):308. doi: 10.3390/toxins15050308. Toxins (Basel). 2023. PMID: 37235343 Free PMC article.
-
Control of Aflatoxigenic Molds by Antagonistic Microorganisms: Inhibitory Behaviors, Bioactive Compounds, Related Mechanisms, and Influencing Factors.Toxins (Basel). 2020 Jan 1;12(1):24. doi: 10.3390/toxins12010024. Toxins (Basel). 2020. PMID: 31906282 Free PMC article. Review.
-
Variation in Occurrence and Aflatoxigenicity of Aspergillus flavus from Two Climatically Varied Regions in Kenya.Toxins (Basel). 2020 Jan 6;12(1):34. doi: 10.3390/toxins12010034. Toxins (Basel). 2020. PMID: 31935922 Free PMC article.
-
Volatiles from Pseudomonas palleroniana Strain B-BH16-1 Suppress Aflatoxin Production and Growth of Aspergillus flavus on Coix lacryma-jobi during Storage.Toxins (Basel). 2023 Jan 14;15(1):77. doi: 10.3390/toxins15010077. Toxins (Basel). 2023. PMID: 36668896 Free PMC article.
References
-
- Brul S, Nussbaum J, Dielbandhoesing SK. Fluorescent probes for wall porosity and membrane integrity in filamentous fungi. J Microbiol Methods. 1997;28:169–178. doi: 10.1016/S0167-7012(97)00975-5. - DOI
-
- Cotty PJ, Bayman P, Egel DS, Elis KS. Agriculture, aflatoxins and Aspergillus. In: Powell KA, Renwick A, Peberdy JF, editors. The genus Aspergillus: from taxonomy and genetics to industrial applications. Plenum Press; New York, NY, USA: 1994. pp. 1–27. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

