CAR T Cell Therapy: A Game Changer in Cancer Treatment
- PMID: 27298832
- PMCID: PMC4889848
- DOI: 10.1155/2016/5474602
CAR T Cell Therapy: A Game Changer in Cancer Treatment
Abstract
The development of novel targeted therapies with acceptable safety profiles is critical to successful cancer outcomes with better survival rates. Immunotherapy offers promising opportunities with the potential to induce sustained remissions in patients with refractory disease. Recent dramatic clinical responses in trials with gene modified T cells expressing chimeric antigen receptors (CARs) in B-cell malignancies have generated great enthusiasm. This therapy might pave the way for a potential paradigm shift in the way we treat refractory or relapsed cancers. CARs are genetically engineered receptors that combine the specific binding domains from a tumor targeting antibody with T cell signaling domains to allow specifically targeted antibody redirected T cell activation. Despite current successes in hematological cancers, we are only in the beginning of exploring the powerful potential of CAR redirected T cells in the control and elimination of resistant, metastatic, or recurrent nonhematological cancers. This review discusses the application of the CAR T cell therapy, its challenges, and strategies for successful clinical and commercial translation.
Figures
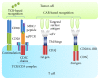


References
-
- Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

