Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNγ Licensing
- PMID: 27299362
- PMCID: PMC5016228
- DOI: 10.1002/stem.2415
Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNγ Licensing
Abstract
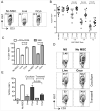
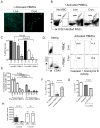
We have previously demonstrated that cryopreservation and thawing lead to altered Mesenchymal stromal cells (MSC) functionalities. Here, we further analyzed MSC's fitness post freeze-thaw. We have observed that thawed MSC can suppress T-cell proliferation when separated from them by transwell membrane and the effect is lost in a MSC:T-cell coculture system. Unlike actively growing MSCs, thawed MSCs were lysed upon coculture with activated autologous Peripheral Blood Mononuclear Cells (PBMCs) and the lysing effect was further enhanced with allogeneic PBMCs. The use of DMSO-free cryoprotectants or substitution of Human Serum Albumin (HSA) with human platelet lysate in freezing media and use of autophagy or caspase inhibitors did not prevent thaw defects. We tested the hypothesis that IFNγ prelicensing before cryobanking can enhance MSC fitness post thaw. Post thawing, IFNγ licensed MSCs inhibit T cell proliferation as well as fresh MSCs and this effect can be blocked by 1-methyl Tryptophan, an Indoleamine 2,3-dioxygenase (IDO) inhibitor. In addition, IFNγ prelicensed thawed MSCs inhibit the degranulation of cytotoxic T cells while IFNγ unlicensed thawed MSCs failed to do so. However, IFNγ prelicensed thawed MSCs do not deploy lung tropism in vivo following intravenous injection as well as fresh MSCs suggesting that IFNγ prelicensing does not fully rescue thaw-induced lung homing defect. We identified reversible and irreversible cryoinjury mechanisms that result in susceptibility to host T-cell cytolysis and affect MSC's cell survival and tissue distribution. The susceptibility of MSC to negative effects of cryopreservation and the potential to mitigate the effects with IFNγ prelicensing may inform strategies to enhance the therapeutic efficacy of MSC in clinical use. Stem Cells 2016;34:2429-2442.
Keywords: Autophagy; Cryopreservation; DMSO; Heat shock; Immune suppression; Indoleamine 2,3-dioxygenase; Mesenchymal stromal cells; T cell responses; Thawing; actin.
© 2016 AlphaMed Press.
Figures





References
-
- Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy. 2015;17:125–127. - PubMed
-
- Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013;13:856–867. - PubMed
-
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

