Serine 707 of APPL1 is Critical for the Synaptic NMDA Receptor-Mediated Akt Phosphorylation Signaling Pathway
- PMID: 27300007
- PMCID: PMC5563782
- DOI: 10.1007/s12264-016-0042-9
Serine 707 of APPL1 is Critical for the Synaptic NMDA Receptor-Mediated Akt Phosphorylation Signaling Pathway
Abstract
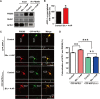
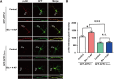
Accumulating evidence indicates that the synaptic activation of N-methyl-D-aspartate receptors (NMDARs) has a neuroprotective effect on neurons. Our previous study demonstrated that APPL1 (adaptor protein containing pleckstrin homology domain, phosphotyrosine-binding domain, and leucine zipper motif) mediates the synaptic activity-dependent activation of PI3K-Akt signaling via coupling this pathway with NMDAR-PSD95 (postsynaptic density protein 95) complexes. However, the molecular mechanism underlying this process is still unknown. In the present study, we investigated the interaction of APPL1 with PSD95 using co-immunocytochemical staining and western blotting. We found that the PDZ2 domain of PSD95 is a binding partner of APPL1. Furthermore, we identified serine 707 of APPL1, a predicted phosphorylation site within the PDZ-binding motif at the C-terminus, as critical for the binding of APPL1 to PSD95, as well as for activation of the Akt signaling pathway during synaptic activity. This suggests that serine 707 of APPL1 is a potential phosphorylation site and may be involved in regulating the neuroprotective Akt signaling pathway that depends on synaptic NMDAR activity.
Keywords: APPL1; Akt; NMDA receptors; Neuroprotection; PSD95.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

