A Farnesyltransferase Acts to Inhibit Ectopic Neurite Formation in C. elegans
- PMID: 27300162
- PMCID: PMC4907426
- DOI: 10.1371/journal.pone.0157537
A Farnesyltransferase Acts to Inhibit Ectopic Neurite Formation in C. elegans
Abstract
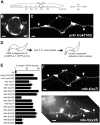
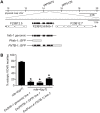
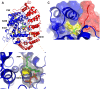
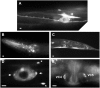
Genetic pathways that regulate nascent neurite formation play a critical role in neuronal morphogenesis. The core planar cell polarity components VANG-1/Van Gogh and PRKL-1/Prickle are involved in blocking inappropriate neurite formation in a subset of motor neurons in C. elegans. A genetic screen for mutants that display supernumerary neurites was performed to identify additional factors involved in this process. This screen identified mutations in fntb-1, the β subunit of farnesyltransferase. We show that fntb-1 is expressed in neurons and acts cell-autonomously to regulate neurite formation. Prickle proteins are known to be post-translationally modified by farnesylation at their C-terminal CAAX motifs. We show that PRKL-1 can be recruited to the plasma membrane in both a CAAX-dependent and CAAX-independent manner but that PRKL-1 can only inhibit neurite formation in a CAAX-dependent manner.
Conflict of interest statement
Figures

Similar articles
-
VANG-1 and PRKL-1 cooperate to negatively regulate neurite formation in Caenorhabditis elegans.PLoS Genet. 2011 Sep;7(9):e1002257. doi: 10.1371/journal.pgen.1002257. Epub 2011 Sep 1. PLoS Genet. 2011. PMID: 21912529 Free PMC article.
-
Dishevelled attenuates the repelling activity of Wnt signaling during neurite outgrowth in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2015 Oct 27;112(43):13243-8. doi: 10.1073/pnas.1518686112. Epub 2015 Oct 12. Proc Natl Acad Sci U S A. 2015. PMID: 26460008 Free PMC article.
-
The planar cell polarity protein VANG-1/Vangl negatively regulates Wnt/β-catenin signaling through a Dvl dependent mechanism.PLoS Genet. 2018 Dec 7;14(12):e1007840. doi: 10.1371/journal.pgen.1007840. eCollection 2018 Dec. PLoS Genet. 2018. PMID: 30532125 Free PMC article.
-
Control of cell polarity and asymmetric division in C. elegans.Curr Top Dev Biol. 2012;101:55-76. doi: 10.1016/B978-0-12-394592-1.00003-X. Curr Top Dev Biol. 2012. PMID: 23140625 Review.
-
Neurite Branching Regulated by Neuronal Cell Surface Molecules in Caenorhabditis elegans.Front Neuroanat. 2020 Aug 21;14:59. doi: 10.3389/fnana.2020.00059. eCollection 2020. Front Neuroanat. 2020. PMID: 32973467 Free PMC article. Review.
Cited by
-
DIP-2 suppresses ectopic neurite sprouting and axonal regeneration in mature neurons.J Cell Biol. 2019 Jan 7;218(1):125-133. doi: 10.1083/jcb.201804207. Epub 2018 Nov 5. J Cell Biol. 2019. PMID: 30396999 Free PMC article.
-
The role of prickle proteins in vertebrate development and pathology.Mol Cell Biochem. 2024 May;479(5):1199-1221. doi: 10.1007/s11010-023-04787-z. Epub 2023 Jun 26. Mol Cell Biochem. 2024. PMID: 37358815 Free PMC article. Review.
References
-
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283: 1931–4. Available: http://www.ncbi.nlm.nih.gov/pubmed/10082468 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

