Bridging Sunitinib Exposure to Time-to-Tumor Progression in Hepatocellular Carcinoma Patients With Mathematical Modeling of an Angiogenic Biomarker
- PMID: 27300260
- PMCID: PMC5131886
- DOI: 10.1002/psp4.12084
Bridging Sunitinib Exposure to Time-to-Tumor Progression in Hepatocellular Carcinoma Patients With Mathematical Modeling of an Angiogenic Biomarker
Abstract
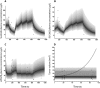
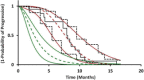
Hepatocellular carcinoma (HCC) is third in cancer-related causes of death worldwide and its treatment is a significant unmet medical need. Sunitinib is a selective tyrosine kinase inhibitor of the angiogenic biomarker: soluble vascular endothelial growth factor receptor-2 (sVEGFR2 ). Sunitinib failed its primary overall survival endpoint in patients with advanced HCC in a phase III trial compared to sorafenib. In the present study, pharmacokinetic-pharmacodynamic modeling was used to link drug-exposure to tumor-growth-inhibition (TGI) and time-to-tumor progression (TTP) through sVEGFR2 dynamics. The results suggest that 1) active drug concentration (i.e., sunitinib and its metabolite) inhibits the release of sVEGFR2 and that such inhibition is associated with TGI, and 2) daily sVEGFR2 exposure is likely a reliable predictor for the TTP in HCC patients. Moreover, the model quantitatively links the dynamics of an angiogenesis biomarker to TTP and accurately predicts observed literature-reported results of placebo treatment.
© 2016 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

Similar articles
-
Antitumor effect of vascular endothelial growth factor inhibitor sunitinib in preclinical models of hepatocellular carcinoma.Eur J Gastroenterol Hepatol. 2012 May;24(5):563-74. doi: 10.1097/MEG.0b013e328350916f. Eur J Gastroenterol Hepatol. 2012. PMID: 22314934
-
Serum C-Telopeptide Collagen Crosslinks and Plasma Soluble VEGFR2 as Pharmacodynamic Biomarkers in a Trial of Sequentially Administered Sunitinib and Cilengitide.Clin Cancer Res. 2015 Nov 15;21(22):5092-9. doi: 10.1158/1078-0432.CCR-15-0427. Epub 2015 Jul 21. Clin Cancer Res. 2015. PMID: 26199386 Free PMC article. Clinical Trial.
-
Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma.Cancer. 2014 Mar 1;120(5):692-701. doi: 10.1002/cncr.28477. Epub 2013 Nov 18. Cancer. 2014. PMID: 24249435 Clinical Trial.
-
Early development of sunitinib in hepatocellular carcinoma.Expert Rev Anticancer Ther. 2009 Jan;9(1):143-50. doi: 10.1586/14737140.9.1.143. Expert Rev Anticancer Ther. 2009. PMID: 19105714 Review.
-
Development of sunitinib in hepatocellular carcinoma: rationale, early clinical experience, and correlative studies.Cancer J. 2009 Jul-Aug;15(4):263-8. doi: 10.1097/PPO.0b013e3181af5e35. Cancer J. 2009. PMID: 19672141 Free PMC article. Review.
Cited by
-
Population Modeling Integrating Pharmacokinetics, Pharmacodynamics, Pharmacogenetics, and Clinical Outcome in Patients With Sunitinib-Treated Cancer.CPT Pharmacometrics Syst Pharmacol. 2017 Sep;6(9):604-613. doi: 10.1002/psp4.12210. Epub 2017 Jul 13. CPT Pharmacometrics Syst Pharmacol. 2017. PMID: 28571114 Free PMC article. Clinical Trial.
-
Multiscale systems pharmacological analysis of everolimus action in hepatocellular carcinoma.J Pharmacokinet Pharmacodyn. 2018 Aug;45(4):607-620. doi: 10.1007/s10928-018-9590-0. Epub 2018 May 3. J Pharmacokinet Pharmacodyn. 2018. PMID: 29725796
-
A Pharmacometric Framework for Axitinib Exposure, Efficacy, and Safety in Metastatic Renal Cell Carcinoma Patients.CPT Pharmacometrics Syst Pharmacol. 2017 Jun;6(6):373-382. doi: 10.1002/psp4.12193. Epub 2017 May 26. CPT Pharmacometrics Syst Pharmacol. 2017. PMID: 28378918 Free PMC article. Clinical Trial.
-
Modeling Hepatocellular Carcinoma Cells Dynamics by Serological and Imaging Biomarkers to Explain the Different Responses to Sorafenib and Regorafenib.Cancers (Basel). 2021 Apr 25;13(9):2064. doi: 10.3390/cancers13092064. Cancers (Basel). 2021. PMID: 33922938 Free PMC article.
-
Progress and Opportunities to Advance Clinical Cancer Therapeutics Using Tumor Dynamic Models.Clin Cancer Res. 2020 Apr 15;26(8):1787-1795. doi: 10.1158/1078-0432.CCR-19-0287. Epub 2019 Dec 23. Clin Cancer Res. 2020. PMID: 31871299 Free PMC article. Review.
References
-
- Parkin, D.M. , Bray, F. , Ferlay, J. & Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 (2005). - PubMed
-
- El‐Serag, H.B. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 37 ( suppl. 2 ), S88–94 (2007). - PubMed
-
- El‐Serag, H.B. & Rudolph, K.L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 (2007). - PubMed
-
- El‐Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 365, 1118–1127 (2011). - PubMed
-
- El‐Serag, H.B. & Mason, A.C. Rising incidence of hepatocellular carcinoma in the United States. N. Engl. J. Med. 340, 745–750 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

