Development of the Pulmonary Arterial Hypertension-Symptoms and Impact (PAH-SYMPACT®) questionnaire: a new patient-reported outcome instrument for PAH
- PMID: 27301413
- PMCID: PMC4908719
- DOI: 10.1186/s12931-016-0388-6
Development of the Pulmonary Arterial Hypertension-Symptoms and Impact (PAH-SYMPACT®) questionnaire: a new patient-reported outcome instrument for PAH
Abstract
Background: Regulators and clinical experts increasingly recognize the importance of incorporating patient-reported outcomes (PROs) in clinical studies of therapies for pulmonary arterial hypertension (PAH). No PAH-specific instruments have been developed to date in accordance with the 2009 FDA guidance for the development of PROs as endpoints in clinical trials. A qualitative research study was conducted to develop a new instrument assessing PAH symptoms and their impacts following the FDA PRO guidance.
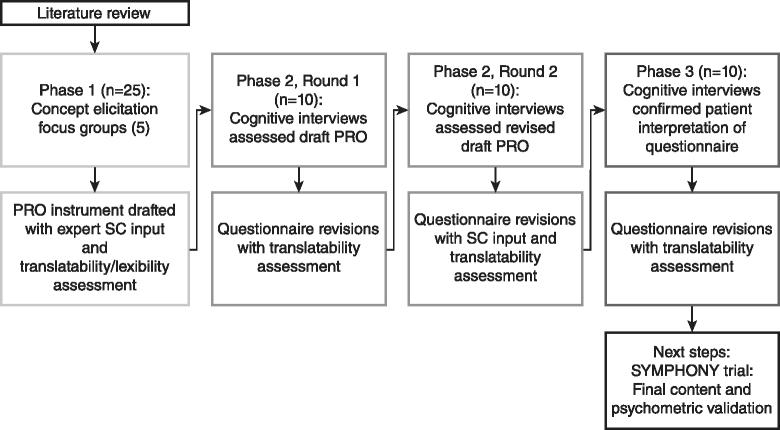
Methods: A cross-sectional study was conducted at 5 centers in the US in symptomatic PAH patients aged 18-80 years. Concept elicitation was based on 5 focus group discussions, after which saturation of emergent concepts was reached. A PRO instrument for PAH symptoms and their impacts was drafted. To assess the appropriateness of items, instructions, response options, and recall periods, 2 rounds of one-on-one cognitive interviews were conducted, with instrument revisions following each round. Additional interviews tested the usability of an electronic version (ePRO). PRO development considered input from an international Steering Committee, and translatability and lexibility assessments.
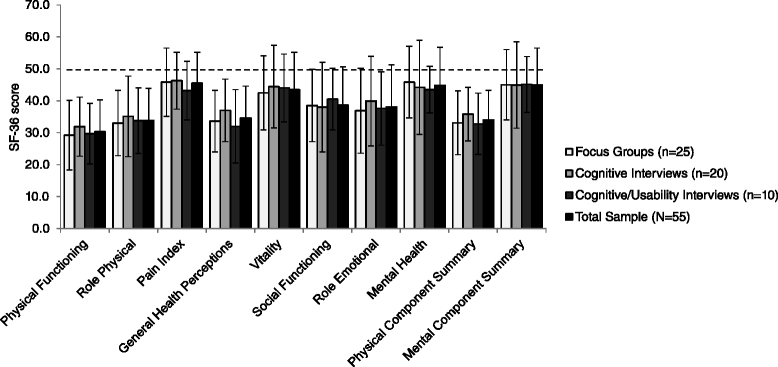
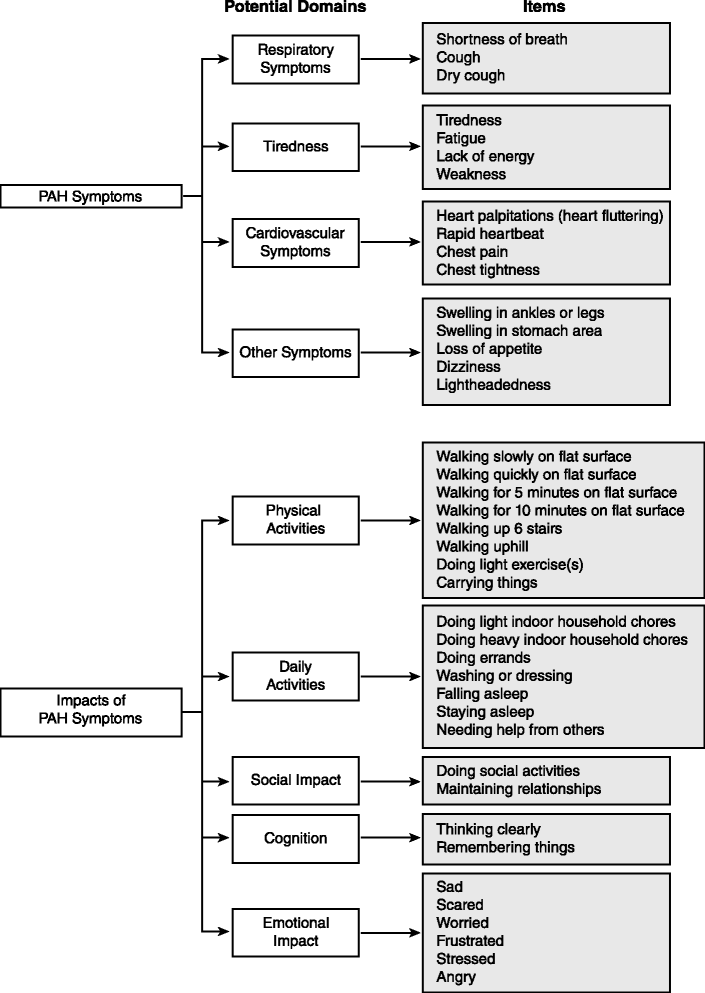
Results: Focus groups comprised 25 patients (5 per group); 20 additional patients participated in cognitive interviews (10 per round); and 10 participated in usability interviews. Participants had a mean ± SD age of 53.1 ± 15.8 years, were predominantly female (93 %), and were diverse in race/ethnicity, WHO functional class (FC I/II: 56 %, III/IV: 44 %), and PAH etiology (idiopathic: 56 %, familial: 2 %, associated: 42 %). The draft PRO instrument (PAH-SYMPACT®) was found to be clear, comprehensive, and relevant to PAH patients in cognitive interviews. Items were organized in a draft conceptual framework with 16 symptom items in 4 domains (respiratory symptoms, tiredness, cardiovascular symptoms, other symptoms) and 25 impact items in 5 domains (physical activities, daily activities, social impact, cognition, emotional impact). The recall period is the past 24 h for symptoms, and the past 7 days for impacts.
Conclusions: The PAH-SYMPACT® was shown to capture symptoms and their impacts relevant to PAH patients, demonstrating content saturation, concept validity, and ePRO usability. Final content and psychometric validation of the instrument will be based on the results of an ongoing Phase IIIb clinical trial in PAH patients.
Keywords: Activities of daily living; Health-related quality of life; Patient-reported outcomes; Pulmonary arterial hypertension; Symptoms.
Figures
Similar articles
-
Psychometric Validation of the Pulmonary Arterial Hypertension-Symptoms and Impact (PAH-SYMPACT) Questionnaire: Results of the SYMPHONY Trial.Chest. 2018 Oct;154(4):848-861. doi: 10.1016/j.chest.2018.04.027. Epub 2018 Apr 26. Chest. 2018. PMID: 29705220 Clinical Trial.
-
Validation of the Pulmonary Arterial Hypertension-Symptoms and Impact for Clinical Use (SYMPACT-CP): a qualitative interview study.BMC Pulm Med. 2025 May 6;25(1):217. doi: 10.1186/s12890-025-03681-2. BMC Pulm Med. 2025. PMID: 40329256 Free PMC article.
-
The CDI-DaySyms: Content Development of a New Patient-Reported Outcome Questionnaire for Symptoms of Clostridium difficile Infection.Value Health. 2018 Apr;21(4):441-448. doi: 10.1016/j.jval.2017.08.3017. Epub 2017 Nov 7. Value Health. 2018. PMID: 29680101 Review.
-
Development and Validation of the FSIQ-RMS: A New Patient-Reported Questionnaire to Assess Symptoms and Impacts of Fatigue in Relapsing Multiple Sclerosis.Value Health. 2019 Apr;22(4):453-466. doi: 10.1016/j.jval.2018.11.007. Epub 2019 Feb 21. Value Health. 2019. PMID: 30975397
-
Development of a new patient-reported outcome measure for facial acne: the Acne Symptom and Impact Scale (ASIS).J Drugs Dermatol. 2014 Mar;13(3):333-40. J Drugs Dermatol. 2014. PMID: 24595580 Review.
Cited by
-
A minimal clinically important difference measured by the Cambridge Pulmonary Hypertension Outcome Review for patients with idiopathic pulmonary arterial hypertension.Pulm Circ. 2021 May 21;11(2):2045894021995055. doi: 10.1177/2045894021995055. eCollection 2021 Apr-Jun. Pulm Circ. 2021. PMID: 34104417 Free PMC article.
-
Cough Sound Detection and Diagnosis Using Artificial Intelligence Techniques: Challenges and Opportunities.IEEE Access. 2021 Jul 15;9:102327-102344. doi: 10.1109/ACCESS.2021.3097559. eCollection 2021. IEEE Access. 2021. PMID: 34786317 Free PMC article.
-
Pulmonary hypertension due to interstitial lung disease or chronic obstructive pulmonary disease: a patient experience study of symptoms and their impact on quality of life.Pulm Circ. 2021 Apr 1;11(2):20458940211005641. doi: 10.1177/20458940211005641. eCollection 2021 Apr-Jun. Pulm Circ. 2021. PMID: 33868642 Free PMC article.
-
Skeletal muscle atrophy in pulmonary arterial hypertension: potential mechanisms and effects of physical exercise.Heart Fail Rev. 2025 Jun 21. doi: 10.1007/s10741-025-10539-6. Online ahead of print. Heart Fail Rev. 2025. PMID: 40542936 Review.
-
The feasibility and value of assessing patient-reported outcomes in pulmonary arterial hypertension.Pulm Circ. 2022 Oct 1;12(4):e12143. doi: 10.1002/pul2.12143. eCollection 2022 Oct. Pulm Circ. 2022. PMID: 36262468 Free PMC article.
References
-
- Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. - DOI - PubMed
-
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous