Virus-like particles derived from Pichia pastoris-expressed dengue virus type 1 glycoprotein elicit homotypic virus-neutralizing envelope domain III-directed antibodies
- PMID: 27301568
- PMCID: PMC4908714
- DOI: 10.1186/s12896-016-0280-y
Virus-like particles derived from Pichia pastoris-expressed dengue virus type 1 glycoprotein elicit homotypic virus-neutralizing envelope domain III-directed antibodies
Abstract
Background: Four antigenically distinct serotypes (1-4) of dengue viruses (DENVs) cause dengue disease. Antibodies to any one DENV serotype have the potential to predispose an individual to more severe disease upon infection with a different DENV serotype. A dengue vaccine must elicit homotypic neutralizing antibodies to all four DENV serotypes to avoid the risk of such antibody-dependent enhancement in the vaccine recipient. This is a formidable challenge as evident from the lack of protective efficacy against DENV-2 by a tetravalent live attenuated dengue vaccine that has completed phase III trials recently. These trial data underscore the need to explore non-replicating subunit vaccine alternatives. Recently, using the methylotrophic yeast Pichia pastoris, we showed that DENV-2 and DENV-3 envelope (E) glycoproteins, expressed in absence of prM, implicated in causing severe dengue disease, self-assemble into virus-like particles (VLPs), which elicit predominantly virus-neutralizing antibodies and confer significant protection against lethal DENV challenge in an animal model. The current study extends this work to a third DENV serotype.
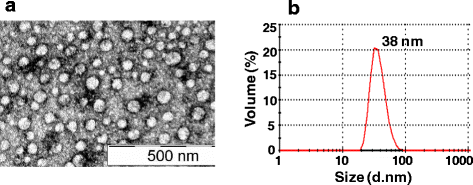
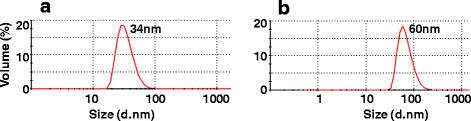
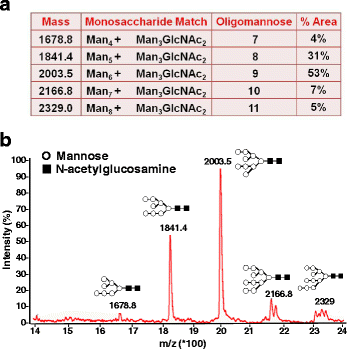
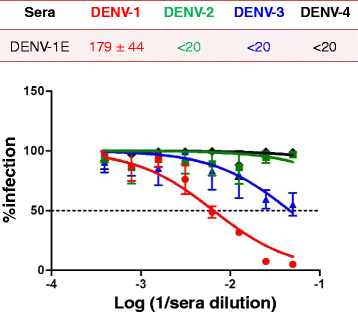
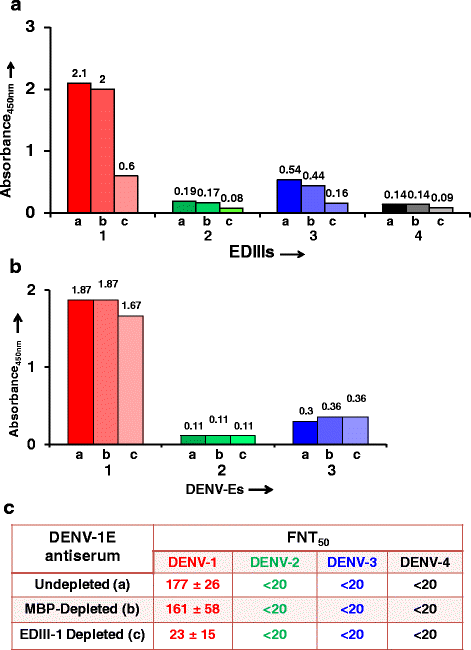
Results: We cloned and expressed DENV-1 E antigen in P. pastoris, and purified it to near homogeneity. Recombinant DENV-1 E underwent post-translational processing, namely, signal peptide cleavage and glycosylation. Purified DENV-1 E self-assembled into stable VLPs, based on electron microscopy and dynamic light scattering analysis. Epitope mapping with monoclonal antibodies revealed that the VLPs retained the overall antigenic integrity of the virion particles despite the absence of prM. Subtle changes accompanied the efficient display of E domain III (EDIII), which contains type-specific neutralizing epitopes. These VLPs were immunogenic, eliciting predominantly homotypic EDIII-directed DENV-1-specific neutralizing antibodies.
Conclusions: This work demonstrates the inherent potential of P. pastoris-expressed DENV-1 E glycoprotein to self-assemble into VLPs eliciting predominantly homotypic neutralizing antibodies. This work justifies an investigation of the last remaining serotype, namely, DENV-4, to assess if it also shares the desirable vaccine potential manifested by the remaining three DENV serotypes. Such efforts could make it possible to envisage the development of a tetravalent dengue vaccine based on VLPs of P. pastoris-expressed E glycoproteins of the four DENV serotypes.
Keywords: Antibody dependent enhancement; Dengue virus; Envelope domain III; Envelope glycoprotein; Neutralizing antibody; Pichia pastoris; Virus-like particles.
Figures

References
-
- Gubler DJ, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Philadelphia: Wolters Kluwer and Lippincott Williams & Wilkins; 2007. pp. 1153–1252.
-
- Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields of Virology. 5. Philadelphia: Wolters Kluwer and Lippincott Williams & Wilkins; 2007. pp. 1101–1152.
-
- WHO Factsheet No117. Dengue and dengue haemorrhagic fever. 2015. http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed 2016 Mar 6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

