Neurotoxicity of the Parkinson Disease-Associated Pesticide Ziram Is Synuclein-Dependent in Zebrafish Embryos
- PMID: 27301718
- PMCID: PMC5089875
- DOI: 10.1289/EHP141
Neurotoxicity of the Parkinson Disease-Associated Pesticide Ziram Is Synuclein-Dependent in Zebrafish Embryos
Abstract
Background: Exposure to the commonly used dithiocarbamate (DTC) pesticides is associated with an increased risk of developing Parkinson disease (PD), although the mechanisms by which they exert their toxicity are not completely understood.
Objective: We studied the mechanisms of ziram's (a DTC fungicide) neurotoxicity in vivo.
Methods: Zebrafish (ZF) embryos were utilized to determine ziram's effects on behavior, neuronal toxicity, and the role of synuclein in its toxicity.
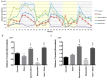
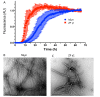
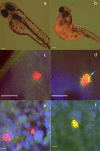
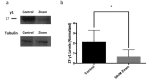
Results: Nanomolar-range concentrations of ziram caused selective loss of dopaminergic (DA) neurons and impaired swimming behavior. Because ziram increases α-synuclein (α-syn) concentrations in rat primary neuronal cultures, we investigated the effect of ziram on ZF γ-synuclein 1 (γ1). ZF express 3 synuclein isoforms, and ZF γ1 appears to be the closest functional homologue to α-syn. We found that recombinant ZF γ1 formed fibrils in vitro, and overexpression of ZF γ1 in ZF embryos led to the formation of neuronal aggregates and neurotoxicity in a manner similar to that of α-syn. Importantly, knockdown of ZF γ1 with morpholinos and disruption of oligomers with the molecular tweezer CLR01 prevented ziram's DA toxicity.
Conclusions: These data show that ziram is selectively toxic to DA neurons in vivo, and this toxicity is synuclein-dependent. These findings have important implications for understanding the mechanisms by which pesticides may cause PD. Citation: Lulla A, Barnhill L, Bitan G, Ivanova MI, Nguyen B, O'Donnell K, Stahl MC, Yamashiro C, Klärner FG, Schrader T, Sagasti A, Bronstein JM. 2016. Neurotoxicity of the Parkinson disease-associated pesticide ziram is synuclein-dependent in zebrafish embryos. Environ Health Perspect 124:1766-1775; http://dx.doi.org/10.1289/EHP141.
Conflict of interest statement
G.B., F.-G.K., and T.S. are co-inventors of U.S. patent no. 8,791,092 and European Patent Application 10 708 075.6, Molecular Tweezers for the Treatment of Amyloid-Related Diseases. The other authors declare they have no actual or potential competing financial interests.
Figures



References
-
- Barnhill LM, Bronstein JM. Pesticides and Parkinson’s disease: is it in your genes? Neurodegener Dis Manag. 2014;4:197–200. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
