Phenotypic Plasticity Regulates Candida albicans Interactions and Virulence in the Vertebrate Host
- PMID: 27303374
- PMCID: PMC4880793
- DOI: 10.3389/fmicb.2016.00780
Phenotypic Plasticity Regulates Candida albicans Interactions and Virulence in the Vertebrate Host
Abstract
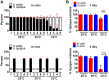
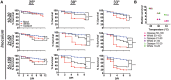
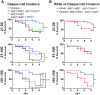
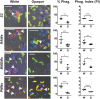
Phenotypic diversity is critical to the lifestyles of many microbial species, enabling rapid responses to changes in environmental conditions. In the human fungal pathogen Candida albicans, cells exhibit heritable switching between two phenotypic states, white and opaque, which yield differences in mating, filamentous growth, and interactions with immune cells in vitro. Here, we address the in vivo virulence properties of the two cell states in a zebrafish model of infection. Multiple attributes were compared including the stability of phenotypic states, filamentation, virulence, dissemination, and phagocytosis by immune cells, and phenotypes equated across three different host temperatures. Importantly, we found that both white and opaque cells could establish a lethal systemic infection. The relative virulence of the two cell types was temperature dependent; virulence was similar at 25°C, but at higher temperatures (30 and 33°C) white cells were significantly more virulent than opaque cells. Despite the difference in virulence, fungal burden, and dissemination were similar between cells in the two states. Additionally, both white and opaque cells exhibited robust filamentation during infection and blocking filamentation resulted in decreased virulence, establishing that this program is critical for pathogenesis in both cell states. Interactions between C. albicans cells and immune cells differed between white and opaque states. Macrophages and neutrophils preferentially phagocytosed white cells over opaque cells in vitro, and neutrophils showed preferential phagocytosis of white cells in vivo. Together, these studies distinguish the properties of white and opaque cells in a vertebrate host, and establish that the two cell types demonstrate both important similarities and key differences during infection.
Keywords: C. albicans; opaque; temperature; virulence; zebrafish model.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

