Cell Wall Composition and Biomass Recalcitrance Differences Within a Genotypically Diverse Set of Brachypodium distachyon Inbred Lines
- PMID: 27303415
- PMCID: PMC4880586
- DOI: 10.3389/fpls.2016.00708
Cell Wall Composition and Biomass Recalcitrance Differences Within a Genotypically Diverse Set of Brachypodium distachyon Inbred Lines
Abstract
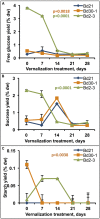
Brachypodium distachyon (Brachypodium) has emerged as a useful model system for studying traits unique to graminaceous species including bioenergy crop grasses owing to its amenability to laboratory experimentation and the availability of extensive genetic and germplasm resources. Considerable natural variation has been uncovered for a variety of traits including flowering time, vernalization responsiveness, and above-ground growth characteristics. However, cell wall composition differences remain underexplored. Therefore, we assessed cell wall-related traits relevant to biomass conversion to biofuels in seven Brachypodium inbred lines that were chosen based on their high level of genotypic diversity as well as available genome sequences and recombinant inbred line (RIL) populations. Senesced stems plus leaf sheaths from these lines exhibited significant differences in acetyl bromide soluble lignin (ABSL), cell wall polysaccharide-derived sugars, hydroxycinnamates content, and syringyl:guaiacyl:p-hydroxyphenyl (S:G:H) lignin ratios. Free glucose, sucrose, and starch content also differed significantly in senesced stems, as did the amounts of sugars released from cell wall polysaccharides (digestibility) upon exposure to a panel of thermochemical pretreatments followed by hydrolytic enzymatic digestion. Correlations were identified between inbred line lignin compositions and plant growth characteristics such as biomass accumulation and heading date (HD), and between amounts of cell wall polysaccharides and biomass digestibility. Finally, stem cell wall p-coumarate and ferulate contents and free-sugars content changed significantly with increased duration of vernalization for some inbred lines. Taken together, these results show that Brachypodium displays substantial phenotypic variation with respect to cell wall composition and biomass digestibility, with some compositional differences correlating with growth characteristics. Moreover, besides influencing HD and biomass accumulation, vernalization was found to affect cell wall composition and free sugars accumulation in some Brachypodium inbred lines, suggesting genetic differences in how vernalization affects carbon flux to polysaccharides. The availability of related RIL populations will allow for the genetic and molecular dissection of this natural variation, the knowledge of which may inform ways to genetically improve bioenergy crop grasses.
Keywords: Pooideae; bioenergy; digestibility; grass; hemicellulose; lignin; polysaccharide; vernalization.
Figures





References
-
- Albersheim P., Nevins D. J., English P. D., Karr A. (1967). A method for the analysis of sugars in plant cell wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 5 340–345. 10.1016/S0008-6215(00)80510-8 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

