Logical Modeling and Dynamical Analysis of Cellular Networks
- PMID: 27303434
- PMCID: PMC4885885
- DOI: 10.3389/fgene.2016.00094
Logical Modeling and Dynamical Analysis of Cellular Networks
Abstract
The logical (or logic) formalism is increasingly used to model regulatory and signaling networks. Complementing these applications, several groups contributed various methods and tools to support the definition and analysis of logical models. After an introduction to the logical modeling framework and to several of its variants, we review here a number of recent methodological advances to ease the analysis of large and intricate networks. In particular, we survey approaches to determine model attractors and their reachability properties, to assess the dynamical impact of variations of external signals, and to consistently reduce large models. To illustrate these developments, we further consider several published logical models for two important biological processes, namely the differentiation of T helper cells and the control of mammalian cell cycle.
Keywords: T cells activation and differentiation; attractors; cell cycle control; discrete dynamics; logical modeling; reachability analysis; regulatory and signaling networks; simulation.
Figures
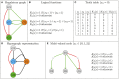


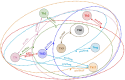

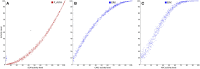
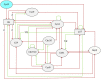
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

