Melanocortin 1 Receptor: Structure, Function, and Regulation
- PMID: 27303435
- PMCID: PMC4885833
- DOI: 10.3389/fgene.2016.00095
Melanocortin 1 Receptor: Structure, Function, and Regulation
Abstract
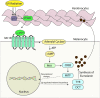
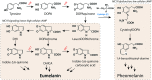
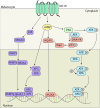
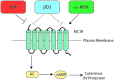
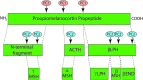
The melanocortin 1 receptor (MC1R) is a melanocytic Gs protein coupled receptor that regulates skin pigmentation, UV responses, and melanoma risk. It is a highly polymorphic gene, and loss of function correlates with a fair, UV-sensitive, and melanoma-prone phenotype due to defective epidermal melanization and sub-optimal DNA repair. MC1R signaling, achieved through adenylyl cyclase activation and generation of the second messenger cAMP, is hormonally controlled by the positive agonist melanocortin, the negative agonist agouti signaling protein, and the neutral antagonist β-defensin 3. Activation of cAMP signaling up-regulates melanin production and deposition in the epidermis which functions to limit UV penetration into the skin and enhances nucleotide excision repair (NER), the genomic stability pathway responsible for clearing UV photolesions from DNA to avoid mutagenesis. Herein we review MC1R structure and function and summarize our laboratory's findings on the molecular mechanisms by which MC1R signaling impacts NER.
Keywords: ASIP; ATR; DNA repair; MC1R; melanocortin; melanocyte; melanoma; βD3.
Figures


References
-
- Abdel-Malek Z. A., Kadekaro A. L., Kavanagh R. J., Todorovic A., Koikov L. N., McNulty J. C., et al. (2006). Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. FASEB J. 20 1561–1563. 10.1096/fj.05-5655fje - DOI - PubMed
-
- Abdel-Malek Z. A., Ruwe A., Kavanagh-Starner R., Kadekaro A. L., Swope V., Haskell-Luevano C., et al. (2009). alpha-MSH tripeptide analogs activate the melanocortin 1 receptor and reduce UV-induced DNA damage in human melanocytes. Pigment Cell Melanoma Res. 22 635–644. 10.1111/j.1755-148X.2009.00598.x - DOI - PubMed
-
- Abdel-Malek Z. A., Scott M. C., Furumura M., Lamoreux M. L., Ollmann M., Barsh G. S., et al. (2001). The melanocortin 1 receptor is the principal mediator of the effects of agouti signaling protein on mammalian melanocytes. J. Cell Sci. 114 1019–1024. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

