Uropathogenic Escherichia coli Metabolite-Dependent Quiescence and Persistence May Explain Antibiotic Tolerance during Urinary Tract Infection
- PMID: 27303698
- PMCID: PMC4863606
- DOI: 10.1128/mSphere.00055-15
Uropathogenic Escherichia coli Metabolite-Dependent Quiescence and Persistence May Explain Antibiotic Tolerance during Urinary Tract Infection
Abstract
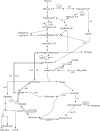
In the present study, it is shown that although Escherichia coli CFT073, a human uropathogenic (UPEC) strain, grows in liquid glucose M9 minimal medium, it fails to grow on glucose M9 minimal medium agar plates seeded with ≤10(6) CFU. The cells on glucose plates appear to be in a "quiescent" state that can be prevented by various combinations of lysine, methionine, and tyrosine. Moreover, the quiescent state is characteristic of ~80% of E. coli phylogenetic group B2 multilocus sequence type 73 strains, as well as 22.5% of randomly selected UPEC strains isolated from community-acquired urinary tract infections in Denmark. In addition, E. coli CFT073 quiescence is not limited to glucose but occurs on agar plates containing a number of other sugars and acetate as sole carbon sources. It is also shown that a number of E. coli CFT073 mini-Tn5 metabolic mutants (gnd, gdhA, pykF, sdhA, and zwf) are nonquiescent on glucose M9 minimal agar plates and that quiescence requires a complete oxidative tricarboxylic acid (TCA) cycle. In addition, evidence is presented that, although E. coli CFT073 quiescence and persistence in the presence of ampicillin are alike in that both require a complete oxidative TCA cycle and each can be prevented by amino acids, E. coli CFT073 quiescence occurs in the presence or absence of a functional rpoS gene, whereas maximal persistence requires a nonfunctional rpoS. Our results suggest that interventions targeting specific central metabolic pathways may mitigate UPEC infections by interfering with quiescence and persistence. IMPORTANCE Recurrent urinary tract infections (UTIs) affect 10 to 40% of women. In up to 77% of those cases, the recurrent infections are caused by the same uropathogenic E. coli (UPEC) strain that caused the initial infection. Upon infection of urothelial transitional cells in the bladder, UPEC appear to enter a nongrowing quiescent intracellular state that is thought to serve as a reservoir responsible for recurrent UTIs. Here, we report that many UPEC strains enter a quiescent state when ≤10(6) CFU are seeded on glucose M9 minimal medium agar plates and show that mutations in several genes involved in central carbon metabolism prevent quiescence, as well as persistence, possibly identifying metabolic pathways involved in UPEC quiescence and persistence in vivo.
Keywords: E. coli persistence; E. coli quiescence; TCA cycle; carbon metabolism; urinary tract infections.
Figures










References
-
- Nicolle LE, Madsen KS, Debeeck GO, Blochlinger E, Borrild N, Bru JP, McKinnon C, O’Doherty B, Spiegel W, Van Balen FA, Menday P.. 2002. Three days of pivmecillinam or norfloxacin for treatment of acute uncomplicated urinary infection in women. Scand J Infect Dis 34:487–492. doi:10.1080/00365540110080728. - DOI - PubMed
-
- Ejrnaes K, Sandvang D, Lundgren B, Ferry S, Holm S, Monsen T, Lundholm R, Frimodt-Moller N.. 2006. Pulsed-field gel electrophoresis typing of Escherichia coli strains from samples collected before and after pivmecillinam or placebo treatment of uncomplicated community-acquired urinary tract infection in women. J Clin Microbiol 44:1776–1781. doi:10.1128/JCM.44.5.1776-1781.2006. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
