Infant Gut Microbiota Development Is Driven by Transition to Family Foods Independent of Maternal Obesity
- PMID: 27303699
- PMCID: PMC4863607
- DOI: 10.1128/mSphere.00069-15
Infant Gut Microbiota Development Is Driven by Transition to Family Foods Independent of Maternal Obesity
Abstract
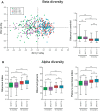
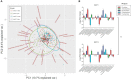
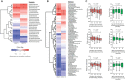
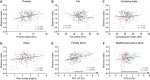
The first years of life are paramount in establishing our endogenous gut microbiota, which is strongly affected by diet and has repeatedly been linked with obesity. However, very few studies have addressed the influence of maternal obesity on infant gut microbiota, which may occur either through vertically transmitted microbes or through the dietary habits of the family. Additionally, very little is known about the effect of diet during the complementary feeding period, which is potentially important for gut microbiota development. Here, the gut microbiotas of two different cohorts of infants, born either of a random sample of healthy mothers (n = 114), or of obese mothers (n = 113), were profiled by 16S rRNA amplicon sequencing. Gut microbiota data were compared to breastfeeding patterns and detailed individual dietary recordings to assess effects of the complementary diet. We found that maternal obesity did not influence microbial diversity or specific taxon abundances during the complementary feeding period. Across cohorts, breastfeeding duration and composition of the complementary diet were found to be the major determinants of gut microbiota development. In both cohorts, gut microbial composition and alpha diversity were thus strongly affected by introduction of family foods with high protein and fiber contents. Specifically, intake of meats, cheeses, and Danish rye bread, rich in protein and fiber, were associated with increased alpha diversity. Our results reveal that the transition from early infant feeding to family foods is a major determinant for gut microbiota development. IMPORTANCE The potential influence of maternal obesity on infant gut microbiota may occur either through vertically transmitted microbes or through the dietary habits of the family. Recent studies have suggested that the heritability of obesity may partly be caused by the transmission of "obesogenic" gut microbes. However, the findings presented here suggest that maternal obesity per se does not affect the overall composition of the gut microbiota and its development after introduction of complementary foods. Rather, progression in complementary feeding is found to be the major determinant for gut microbiota establishment. Expanding our understanding of the influence of complementary diet on the development and establishment of the gut microbiota will provide us with the knowledge to tailor a beneficial progression of our intestinal microbial community.
Keywords: 16S rRNA sequencing; breastfeeding; complementary diet; family foods; infant gut microbiota; maternal obesity.
Figures


References
-
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. doi: 10.1126/science.1208344. - DOI - PMC - PubMed
-
- Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, Scott JA, Doré J, Edwards CA, INFABIO Team . 2011. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 157:1385–1392. doi: 10.1099/mic.0.042143-0. - DOI - PubMed
-
- Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL, CHILD Study Investigators . 2013. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 185:385–394. doi: 10.1503/cmaj.121189. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
