Strain-Level Differences in Porphyrin Production and Regulation in Propionibacterium acnes Elucidate Disease Associations
- PMID: 27303708
- PMCID: PMC4863617
- DOI: 10.1128/mSphere.00023-15
Strain-Level Differences in Porphyrin Production and Regulation in Propionibacterium acnes Elucidate Disease Associations
Abstract
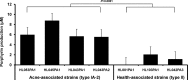
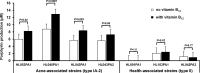
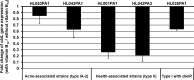
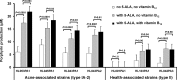
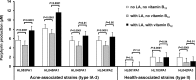
Propionibacterium acnes is an important skin commensal, but it is also considered a pathogenic factor in several diseases including acne vulgaris, the most common skin disease. While previous studies have revealed P. acnes strain-level differences in health and disease associations, the underlying molecular mechanisms remain unknown. Recently, we demonstrated that vitamin B12 supplementation increases P. acnes production of porphyrins, a group of proinflammatory metabolites important in acne development (D. Kang, B. Shi, M. C. Erfe, N. Craft, and H. Li, Sci. Transl. Med. 7:293ra103, 2015, doi:10.1126/scitranslmed.aab2009). In this study, we compared the porphyrin production and regulation of multiple P. acnes strains. We revealed that acne-associated type IA-2 strains inherently produced significantly higher levels of porphyrins, which were further enhanced by vitamin B12 supplementation. On the other hand, health-associated type II strains produced low levels of porphyrins and did not respond to vitamin B12. Using a small-molecule substrate and inhibitor, we demonstrated that porphyrin biosynthesis was modulated at the metabolic level. We identified a repressor gene (deoR) of porphyrin biosynthesis that was carried in all health-associated type II strains, but not in acne-associated type IA-2 strains. The expression of deoR suggests additional regulation of porphyrin production at the transcriptional level in health-associated strains. Our findings provide one potential molecular mechanism for the different contributions of P. acnes strains to skin health and disease and support the role of vitamin B12 in acne pathogenesis. Our study emphasizes the importance of understanding the role of the commensal microbial community in health and disease at the strain level and suggests potential utility of health-associated P. acnes strains in acne treatment. IMPORTANCE Propionibacterium acnes is a dominant bacterium residing on skin, and it has been thought to play a causal role in several diseases including acne, a common skin disease affecting more than 80% of people worldwide. While specific strains of P. acnes have been associated with either disease or healthy skin, the mechanisms remain unclear. Recently, we showed that vitamin B12 supplementation increased porphyrin production in P. acnes, leading to acne development (D. Kang, B. Shi, M. C. Erfe, N. Craft, and H. Li, Sci. Transl. Med. 7:293ra103, 2015, doi:10.1126/scitranslmed.aab2009). Here, we reveal that the levels of porphyrin production and vitamin B12 regulation are different between acne- and health-associated strains, suggesting a potential molecular mechanism for disease-associated strains in acne pathogenesis and for health-associated strains in skin health. This study highlights the importance of understanding the strain-level differences of the human microbiota in disease pathogenesis. Our findings also suggest the porphyrin biosynthesis pathway as a candidate drug target and use of health-associated strains as potential probiotics in novel acne therapeutics.
Keywords: 5-aminolevulinic acid; Propionibacterium acnes; acne; levulinic acid; porphyrin; strain; vitamin B12.
Figures

References
-
- Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, Seltmann H, Patrick S, Zouboulis CC, Kemény L. 2006. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect 8:2195–2205. doi:10.1016/j.micinf.2006.04.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

