Crystal structures of two monomeric triosephosphate isomerase variants identified via a directed-evolution protocol selecting for L-arabinose isomerase activity
- PMID: 27303904
- PMCID: PMC4909251
- DOI: 10.1107/S2053230X16007548
Crystal structures of two monomeric triosephosphate isomerase variants identified via a directed-evolution protocol selecting for L-arabinose isomerase activity
Abstract
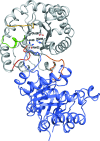
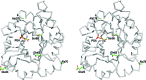
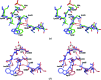
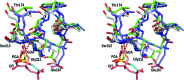
The crystal structures are described of two variants of A-TIM: Ma18 (2.7 Å resolution) and Ma21 (1.55 Å resolution). A-TIM is a monomeric loop-deletion variant of triosephosphate isomerase (TIM) which has lost the TIM catalytic properties. Ma18 and Ma21 were identified after extensive directed-evolution selection experiments using an Escherichia coli L-arabinose isomerase knockout strain expressing a randomly mutated A-TIM gene. These variants facilitate better growth of the Escherichia coli selection strain in medium supplemented with 40 mM L-arabinose. Ma18 and Ma21 differ from A-TIM by four and one point mutations, respectively. Ma18 and Ma21 are more stable proteins than A-TIM, as judged from CD melting experiments. Like A-TIM, both proteins are monomeric in solution. In the Ma18 crystal structure loop 6 is open and in the Ma21 crystal structure loop 6 is closed, being stabilized by a bound glycolate molecule. The crystal structures show only small differences in the active site compared with A-TIM. In the case of Ma21 it is observed that the point mutation (Q65L) contributes to small structural rearrangements near Asn11 of loop 1, which correlate with different ligand-binding properties such as a loss of citrate binding in the active site. The Ma21 structure also shows that its Leu65 side chain is involved in van der Waals interactions with neighbouring hydrophobic side-chain moieties, correlating with its increased stability. The experimental data suggest that the increased stability and solubility properties of Ma21 and Ma18 compared with A-TIM cause better growth of the selection strain when coexpressing Ma21 and Ma18 instead of A-TIM.
Keywords: TIM barrel; enzyme engineering; non-natural enzymes; structure-based rational design; triosephosphate isomerase.
Figures


Similar articles
-
Structure-based directed evolution of a monomeric triosephosphate isomerase: toward a pentose sugar isomerase.Protein Eng Des Sel. 2015 Jun;28(6):187-97. doi: 10.1093/protein/gzv010. Epub 2015 Mar 11. Protein Eng Des Sel. 2015. PMID: 25767111
-
Structural studies show that the A178L mutation in the C-terminal hinge of the catalytic loop-6 of triosephosphate isomerase (TIM) induces a closed-like conformation in dimeric and monomeric TIM.Acta Crystallogr D Biol Crystallogr. 2008 Feb;64(Pt 2):178-88. doi: 10.1107/S0907444907059021. Epub 2008 Jan 16. Acta Crystallogr D Biol Crystallogr. 2008. PMID: 18219118
-
Crystallographic binding studies with an engineered monomeric variant of triosephosphate isomerase.Acta Crystallogr D Biol Crystallogr. 2010 Aug;66(Pt 8):934-44. doi: 10.1107/S0907444910025710. Epub 2010 Jul 14. Acta Crystallogr D Biol Crystallogr. 2010. PMID: 20693693
-
The TIM-barrel fold: a versatile framework for efficient enzymes.FEBS Lett. 2001 Mar 16;492(3):193-8. doi: 10.1016/s0014-5793(01)02236-0. FEBS Lett. 2001. PMID: 11257493 Review.
-
Triosephosphate isomerase: a highly evolved biocatalyst.Cell Mol Life Sci. 2010 Dec;67(23):3961-82. doi: 10.1007/s00018-010-0473-9. Epub 2010 Aug 7. Cell Mol Life Sci. 2010. PMID: 20694739 Free PMC article. Review.
References
-
- Alahuhta, M., Casteleijn, M. G., Neubauer, P. & Wierenga, R. K. (2008). Acta Cryst. D64, 178–188. - PubMed
-
- Alahuhta, M., Salin, M., Casteleijn, M. G., Kemmer, C., El-Sayed, I., Augustyns, K., Neubauer, P. & Wierenga, R. K. (2008). Protein Eng. Des. Sel. 21, 257–266. - PubMed
-
- Blomberg, R., Kries, H., Pinkas, D. M., Mittl, P. R., Grütter, M. G., Privett, H. K., Mayo, S. L. & Hilvert, D. (2013). Nature (London), 503, 418–421. - PubMed
-
- Borchert, T. V., Abagyan, R., Kishan, K. R., Zeelen, J. & Wierenga, R. (1993). Structure, 1, 205–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

