Liver imaging reporting and data system (LI-RADS) version 2014: understanding and application of the diagnostic algorithm
- PMID: 27304548
- PMCID: PMC4946409
- DOI: 10.3350/cmh.2016.0028
Liver imaging reporting and data system (LI-RADS) version 2014: understanding and application of the diagnostic algorithm
Abstract
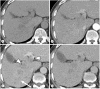
Liver Imaging Reporting and Data System (LI-RADS) is a system for interpreting and reporting of computed tomography and magnetic resonance imaging of the liver in patients at risk for hepatocellular carcinoma (HCC). LI-RADS has been developed to address the limitations of prior imaging-based criteria including the lack of established consensus regarding the exact definitions of imaging features, binary categorization (either definite or not definite HCC), and failure to consider non-HCC malignancies. One of the most important goals of LI-RADS is to facilitate clear communication between all the personnel involved in the diagnosis and treatment of HCC, such as radiologists, hepatologists, surgeons, and pathologists. Therefore, clinicians should also be familiar with LI-RADS. This article reviews the LI-RADS diagnostic algorithm, and the definitions and management implications of LI-RADS categories.
Keywords: Algorithms; Carcinoma; Diagnosis; Guideline; Hepatocellular.
Conflict of interest statement
Figures

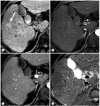
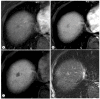
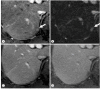
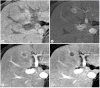

References
-
- EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. - PubMed
-
- Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. - PubMed
-
- Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology. 2014;87:S7–S21. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

