Effects of Pitavastatin on Lipid Profiles in HIV-Infected Patients with Dyslipidemia and Receiving Atazanavir/Ritonavir: A Randomized, Double-Blind, Crossover Study
- PMID: 27304841
- PMCID: PMC4909195
- DOI: 10.1371/journal.pone.0157531
Effects of Pitavastatin on Lipid Profiles in HIV-Infected Patients with Dyslipidemia and Receiving Atazanavir/Ritonavir: A Randomized, Double-Blind, Crossover Study
Abstract
Background: Dyslipidemia as a risk factor of cardiovascular disease is common especially in HIV-infected patients who are using protease inhibitors (PIs) including atazanavir. Pitavastatin has less drug-drug interactions and demonstrable efficacy in decreasing lipid levels in non HIV-infected individuals.
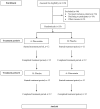
Materials and methods: This study was a randomized, double-blind, crossover study comparing the safety and efficacy of pitavastatin vs placebo in HIV-infected patients with dyslipidemia and receiving atazanavir/ritonavir (ATV/r). Patients were randomized to receive either placebo or pitavastatin for 12 weeks. The follow-up visits were every 4 weeks until the end of the study.
Results: A total of 12 HIV-infected patients were enrolled to each study group. Of all, 14 (58%) patients were men and mean (standard deviation, SD) age was 48.1 (1.8) years. At 12 weeks of treatment with pitavastatin compared to placebo; mean [95% confidence interval (CI)] total cholesterol (TC) was 207 (187.3, 226.8) mg/dL vs 246.3 (226.5, 266) mg/dL (p <0.001); mean (95% CI) triglyceride (TG) was 351.3 (193.2, 509.4) mg/dL vs 279.1 (121, 437.2) mg/dL (p = 0.269); mean (95% CI) high density lipoprotein (HDL) was 45.3 (40.4, 50.2) mg/dL vs 44.2 (39.3, 49.1) mg/dL (p = 0.354); and mean (95% CI) low density lipoprotein (LDL) was 113.2 (100.4, 126) mg/dL vs 145.6 (132.8, 158.4) mg/dL (p <0.001). Mean liver enzyme and median creatine phosphokinase levels were not statistically significant between patients receiving placebo and pitavastatin.
Conclusions: Pitavastatin decreases TC and LDL level at 12 weeks significantly and shows indifferent in hepatotoxicity and creatine phosphokinase levels compared to those of placebo. Thus, pitavastatin can be a good option of lipid-lowering agent in HIV-infected patients who are receiving ATV/r.
Trial registration: ClinicalTrials.gov NCT02442700.
Conflict of interest statement
Figures
References
-
- Estrada V, Portilla J. Dyslipidemia related to antiretroviral therapy. AIDS Rev. 2011;13: 49–56. - PubMed
-
- Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis. 2006;185: 1–11. - PubMed
-
- Guardiola M, Ferre R, Salazar J, Alonso-Villaverde C, Coll B, Parra S, et al. Protease inhibitor-associated dyslipidemia in HIV- infected patients is strongly influenced by the APOA5-1131T->C gene variation. Clin Chem. 2006;52: 1914–1919. - PubMed
-
- Dube PM, Stein JH, Aberg JA, Fichtenbaum CJ, Gerber JG, Tashima KT, et al. Guidelines for the evaluation and management of dyslipidemia in Human Immunodeficiency Virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medicine Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37: 613–627. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous