The Phosphoinosotide 3-Kinase Catalytic Subunit p110α is Required for Normal Lens Growth
- PMID: 27304846
- PMCID: PMC4928694
- DOI: 10.1167/iovs.16-19607
The Phosphoinosotide 3-Kinase Catalytic Subunit p110α is Required for Normal Lens Growth
Abstract
Purpose: Signal transduction pathways influence lens growth, but little is known about the role(s) of the class 1A phosphoinositide 3-kinases (PI3Ks). To further investigate how signaling regulates lens growth, we generated and characterized mice in which the p110α and p110β catalytic subunits of PI3K were conditionally deleted in the mouse lens.
Methods: Floxed alleles of the catalytic subunits of PI3K were conditionally deleted in the lens by using MLR10-cre transgenic mice. Lenses of age-matched animals were dissected and photographed. Postnatal lenses were fixed, paraffin embedded, sectioned, and stained with hematoxylin-eosin. Cell proliferation was quantified by labeling S-phase cells in intact lenses with 5-ethynyl-2'-deoxyuridine. Protein kinase B (AKT) activation was examined by Western blotting.
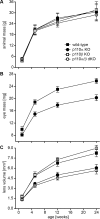
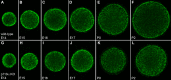
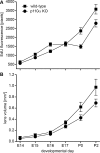
Results: Lens-specific deletion of p110α resulted in a significant reduction of eye and lens size, without compromising lens clarity. Conditional knockout of p110β had no effect on lens size or clarity, and deletion of both the p110α and p110β subunits resulted in a phenotype that resembled the p110α single-knockout phenotype. Levels of activated AKT were decreased more in p110α- than in p110β-deficient lenses. A significant reduction in proliferating cells in the germinative zone was observed on postnatal day 0 in p110α knockout mice, which was temporally correlated with decreased lens volume.
Conclusions: These data suggest that the class 1A PI3K signaling pathway plays an important role in the regulation of lens size by influencing the extent and spatial location of cell proliferation in the perinatal period.
Figures




Similar articles
-
Double Deletion of PI3K and PTEN Modifies Lens Postnatal Growth and Homeostasis.Cells. 2022 Aug 30;11(17):2708. doi: 10.3390/cells11172708. Cells. 2022. PMID: 36078116 Free PMC article.
-
Role of phosphoinositide 3-OH kinase p110β in skeletal myogenesis.Mol Cell Biol. 2015 Apr;35(7):1182-96. doi: 10.1128/MCB.00550-14. Epub 2015 Jan 20. Mol Cell Biol. 2015. PMID: 25605332 Free PMC article.
-
p110α and p110β isoforms of PI3K are involved in protection against H2O2 induced oxidative stress in cancer cells.Breast Cancer. 2019 May;26(3):378-385. doi: 10.1007/s12282-018-0933-x. Epub 2018 Nov 29. Breast Cancer. 2019. PMID: 30499025
-
Class I Phosphoinositide 3-Kinase PIK3CA/p110α and PIK3CB/p110β Isoforms in Endometrial Cancer.Int J Mol Sci. 2018 Dec 7;19(12):3931. doi: 10.3390/ijms19123931. Int J Mol Sci. 2018. PMID: 30544563 Free PMC article. Review.
-
p110α and p110β isoforms of PI3K signaling: are they two sides of the same coin?FEBS Lett. 2016 Sep;590(18):3071-82. doi: 10.1002/1873-3468.12377. Epub 2016 Sep 12. FEBS Lett. 2016. PMID: 27552098 Review.
Cited by
-
Altered Cell Clusters and Upregulated Aqp1 in Connexin 50 Knockout Lens Epithelium.Invest Ophthalmol Vis Sci. 2024 Sep 3;65(11):27. doi: 10.1167/iovs.65.11.27. Invest Ophthalmol Vis Sci. 2024. PMID: 39287589 Free PMC article.
-
Lens differentiation is controlled by the balance between PDGF and FGF signaling.PLoS Biol. 2019 Feb 4;17(2):e3000133. doi: 10.1371/journal.pbio.3000133. eCollection 2019 Feb. PLoS Biol. 2019. PMID: 30716082 Free PMC article.
-
A full lifespan model of vertebrate lens growth.R Soc Open Sci. 2017 Jan 18;4(1):160695. doi: 10.1098/rsos.160695. eCollection 2017 Jan. R Soc Open Sci. 2017. PMID: 28280571 Free PMC article.
-
Suppression of PI3K signaling is linked to autophagy activation and the spatiotemporal induction of the lens organelle free zone.Exp Cell Res. 2022 Mar 15;412(2):113043. doi: 10.1016/j.yexcr.2022.113043. Epub 2022 Jan 29. Exp Cell Res. 2022. PMID: 35101390 Free PMC article.
-
PI3K Isoform-Specific Regulation of Leader and Follower Cell Function for Collective Migration and Proliferation in Response to Injury.Cells. 2022 Nov 7;11(21):3515. doi: 10.3390/cells11213515. Cells. 2022. PMID: 36359913 Free PMC article.
References
-
- Ilic N,, Roberts TM. Comparing the roles of the p110alpha and p110beta isoforms of PI3K in signaling and cancer. Curr Top Microbiol Immunol. 2010; 347: 55–77. - PubMed
-
- Domin J,, Waterfield MD. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997; 410: 91–95. - PubMed
-
- Vanhaesebroeck B,, Guillermet-Guibert J,, Graupera M,, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010; 11: 329–341. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002; 296: 1655–1657. - PubMed
-
- Engelman JA,, Luo J,, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006; 7: 606–619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

