Naturally Inspired Peptide Leads: Alanine Scanning Reveals an Actin-Targeting Thiazole Analogue of Bisebromoamide
- PMID: 27304907
- PMCID: PMC5096027
- DOI: 10.1002/cbic.201600257
Naturally Inspired Peptide Leads: Alanine Scanning Reveals an Actin-Targeting Thiazole Analogue of Bisebromoamide
Abstract
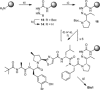
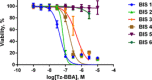
Systematic alanine scanning of the linear peptide bisebromoamide (BBA), isolated from a marine cyanobacterium, was enabled by solid-phase peptide synthesis of thiazole analogues. The analogues have comparable cytotoxicity (nanomolar) to that of BBA, and cellular morphology assays indicated that they target the actin cytoskeleton. Pathway inhibition in human colon tumour (HCT116) cells was explored by reverse phase protein array (RPPA) analysis, which showed a dose-dependent response in IRS-1 expression. Alanine scanning reveals a structural dependence to the cytotoxicity, actin targeting and pathway inhibition, and allows a new readily synthesised lead to be proposed.
Keywords: alanine scan; cell morphology; nonribosomal peptides; reverse phase protein array; solid-phase synthesis; structure-activity relationships.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures



References
-
- None
-
- None
-
- Walsh C. T., Nat. Prod. Rep. 2016, 33, 127–135; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

