Astrocytes Enhance Streptococcus suis-Glial Cell Interaction in Primary Astrocyte-Microglial Cell Co-Cultures
- PMID: 27304968
- PMCID: PMC4931394
- DOI: 10.3390/pathogens5020043
Astrocytes Enhance Streptococcus suis-Glial Cell Interaction in Primary Astrocyte-Microglial Cell Co-Cultures
Abstract
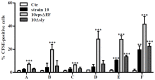
Streptococcus (S.) suis infections are the most common cause of meningitis in pigs. Moreover, S. suis is a zoonotic pathogen, which can lead to meningitis in humans, mainly in adults. We assume that glial cells may play a crucial role in host-pathogen interactions during S. suis infection of the central nervous system. Glial cells are considered to possess important functions during inflammation and injury of the brain in bacterial meningitis. In the present study, we established primary astrocyte-microglial cell co-cultures to investigate interactions of S. suis with glial cells. For this purpose, microglial cells and astrocytes were isolated from new-born mouse brains and characterized by flow cytometry, followed by the establishment of astrocyte and microglial cell mono-cultures as well as astrocyte-microglial cell co-cultures. In addition, we prepared microglial cell mono-cultures co-incubated with uninfected astrocyte mono-culture supernatants and astrocyte mono-cultures co-incubated with uninfected microglial cell mono-culture supernatants. After infection of the different cell cultures with S. suis, bacteria-cell association was mainly observed with microglial cells and most prominently with a non-encapsulated mutant of S. suis. A time-dependent induction of NO release was found only in the co-cultures and after co-incubation of microglial cells with uninfected supernatants of astrocyte mono-cultures mainly after infection with the capsular mutant. Only moderate cytotoxic effects were found in co-cultured glial cells after infection with S. suis. Taken together, astrocytes and astrocyte supernatants increased interaction of microglial cells with S. suis. Astrocyte-microglial cell co-cultures are suitable to study S. suis infections and bacteria-cell association as well as NO release by microglial cells was enhanced in the presence of astrocytes.
Keywords: NO release; S. suis; astrocytes; bacteria-cell-association; co-cultures; microglial cells.
Figures




References
-
- Reams R.Y., Glickman L.T., Harrington D.D., Thacker H.L., Bowersock T.L. Streptococcus suis infection in swine: A retrospective study of 256 cases. Part ii. Clinical signs, gross and microscopic lesions, and coexisting microorganisms. J. Vet. Diagn. Investig. 1994;6:326–334. doi: 10.1177/104063879400600308. - DOI - PubMed
-
- Straw B.E. Diseases of Swine. 9th ed. Blackwell Publisher; Ames, IA, USA: 2006.
LinkOut - more resources
Full Text Sources
Other Literature Sources

