Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis
- PMID: 27304991
- PMCID: PMC5082781
- DOI: 10.1080/15548627.2016.1190071
Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis
Abstract
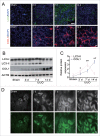
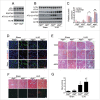
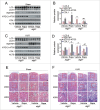
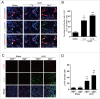
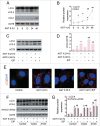
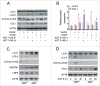
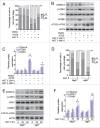
Macroautophagy/autophagy protects against cellular stress. Renal sublethal injury-triggered tubular epithelial cell cycle arrest at G2/M is associated with interstitial fibrosis. However, the role of autophagy in renal fibrosis is elusive. Here, we hypothesized that autophagy activity in tubular epithelial cells is pivotal for inhibition of cell cycle G2/M arrest and subsequent fibrogenic response. In both renal epithelial cells stimulated by angiotensin II (AGT II) and the murine kidney after unilateral ureteral obstruction (UUO), we observed that occurrence of autophagy preceded increased production of COL1 (collagen, type I). Pharmacological enhancement of autophagy by rapamycin suppressed COL1 accumulation and renal fibrosis. In contrast, genetic ablation of autophagy by proximal tubular epithelial cell-specific deletion of Atg5, with reduction of the LC3-II protein level and degradation of SQSTM1/p62, showed marked cell cycle arrest at the G2/M phase, robust COL1 deposition, and severe interstitial fibrosis in a UUO model, as compared with wild-type mice. In vitro, AGT II exposure triggered autophagy preferentially in the G1/S phase, and increased COL1 expression in the G2/M phase in renal epithelial cells. Stimulation of Atg5-deficient primary proximal tubular cells with AGT II also resulted in elevated G2/M arrest and COL1 production. Pharmacological or genetic inhibition of autophagy increased AGT II-mediated G2/M arrest. Enhanced expression of ATG5, but not the autophagy-deficient ATG5 mutant K130R, rescued the G2/M arrest, suggesting the regulation of cell cycle progression by ATG5 is autophagy dependent. In conclusion, Atg5-mediated autophagy in proximal epithelial cells is a critical host-defense mechanism that prevents renal fibrosis by blocking G2/M arrest.
Keywords: ATG5; autophagy; cell cycle; proximal tubular epithelial cells; renal fibrosis.
Figures



References
-
- Radhakrishnan J, Remuzzi G, Saran R, Williams DE, Rios-Burrows N, Powe N, Bruck K, Wanner C, Stel VS, Venuthurupalli SK, et al.. Taming the chronic kidney disease epidemic: a global view of surveillance efforts. Kidney Int 2014; 86:246–50; PMID:24897034; http://dx.doi.org/10.1038/ki.2014.190 - DOI - PMC - PubMed
-
- Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant 2015; 30(4):575–83; PMID:25016609; http://dx.doi.org/10.1093/ndt/gfu230 - DOI - PMC - PubMed
-
- Thomasova D, Anders HJ. Cell cycle control in the kidney. Nephrol Dial Transplant 2015; 30(10):1622–30; PMID:25538161; http://dx.doi.org/10.1093/ndt/gfu395 - DOI - PubMed
-
- Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 2010; 16:535–43, 1p-143p; http://dx.doi.org/10.1038/nm.2144 - DOI - PMC - PubMed
-
- Hale AN, Ledbetter DJ, Gawriluk TR, Rucker ER. Autophagy: regulation and role in development. Autophagy 2013; 9:951–72; PMID:24121596; http://dx.doi.org/10.4161/auto.24273 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
