Rapid, Culture-Free Detection of Staphylococcus aureus Bacteremia
- PMID: 27305148
- PMCID: PMC4909304
- DOI: 10.1371/journal.pone.0157234
Rapid, Culture-Free Detection of Staphylococcus aureus Bacteremia
Abstract
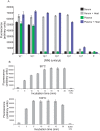
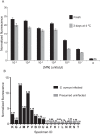
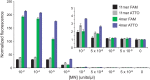
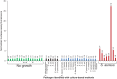
S. aureus bacteremia (SAB) is a common condition with high rates of morbidity and mortality. Current methods used to diagnose SAB take at least a day, and often longer. Patients with suspected bacteremia must therefore be empirically treated, often unnecessarily, while assay results are pending. In this proof-of-concept study, we describe an inexpensive assay that detects SAB via the detection of micrococcal nuclease (an enzyme secreted by S. aureus) in patient plasma samples in less than three hours. In total, 17 patient plasma samples from culture-confirmed S. aureus bacteremic individuals were tested. 16 of these yielded greater nuclease assay signals than samples from uninfected controls or individuals with non-S. aureus bacteremia. These results suggest that a nuclease-detecting assay may enable the rapid and inexpensive diagnosis of SAB, which is expected to substantially reduce the mortality and morbidity that result from this condition.
Conflict of interest statement
Figures
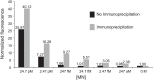
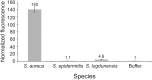
Similar articles
-
Rapid molecular testing for Staphylococcus aureus bacteraemia improves clinical management.J Med Microbiol. 2020 Apr;69(4):552-557. doi: 10.1099/jmm.0.001171. J Med Microbiol. 2020. PMID: 32141812
-
Molecular detection of Staphylococcus aureus in urine in patients with S. aureus bacteremia: an exploratory study.Eur J Clin Microbiol Infect Dis. 2025 Jan;44(1):37-43. doi: 10.1007/s10096-024-04969-7. Epub 2024 Oct 31. Eur J Clin Microbiol Infect Dis. 2025. PMID: 39480590 Free PMC article.
-
Evaluation of three methods for the rapid identification of Staphylococcus aureus in blood cultures.Diagn Microbiol Infect Dis. 1994 May;19(1):5-8. doi: 10.1016/0732-8893(94)90043-4. Diagn Microbiol Infect Dis. 1994. PMID: 7956013
-
The clinical significance of concomitant bacteriuria in patients with Staphylococcus aureus bacteremia. A review and meta-analysis.Infect Dis (Lond). 2018 Sep;50(9):648-659. doi: 10.1080/23744235.2018.1445280. Epub 2018 Feb 28. Infect Dis (Lond). 2018. PMID: 29489435 Review.
-
Staphylococcus aureus bacteremia and endocarditis.Cardiol Clin. 2003 May;21(2):219-33, vii. doi: 10.1016/s0733-8651(03)00030-4. Cardiol Clin. 2003. PMID: 12874895 Review.
Cited by
-
Ex Vivo Tracer Efficacy in Optical Imaging of Staphylococcus Aureus Nuclease Activity.Sci Rep. 2018 Jan 22;8(1):1305. doi: 10.1038/s41598-018-19289-y. Sci Rep. 2018. PMID: 29358617 Free PMC article.
-
Colorimetric Detection of Staphylococcus aureus Contaminated Solutions without Purification.Bioconjug Chem. 2017 Jan 18;28(1):183-193. doi: 10.1021/acs.bioconjchem.6b00571. Epub 2016 Dec 2. Bioconjug Chem. 2017. PMID: 28095683 Free PMC article.
-
Rapid Detection of Urinary Tract Infections via Bacterial Nuclease Activity.Mol Ther. 2017 Jun 7;25(6):1353-1362. doi: 10.1016/j.ymthe.2017.03.015. Epub 2017 Apr 5. Mol Ther. 2017. PMID: 28391960 Free PMC article.
-
A fluorogenic micrococcal nuclease-based probe for fast detection and optical imaging of Staphylococcus aureus in prosthetic joint and fracture-related infections.Eur J Nucl Med Mol Imaging. 2024 Aug;51(10):2988-2997. doi: 10.1007/s00259-023-06499-4. Epub 2023 Nov 14. Eur J Nucl Med Mol Imaging. 2024. PMID: 37962617 Free PMC article.
-
Rapid and Sensitive Detection of Breast Cancer Cells in Patient Blood with Nuclease-Activated Probe Technology.Mol Ther Nucleic Acids. 2017 Sep 15;8:542-557. doi: 10.1016/j.omtn.2017.08.004. Epub 2017 Aug 12. Mol Ther Nucleic Acids. 2017. PMID: 28918054 Free PMC article.
References
-
- Diekema DJ, Pfaller MA, Jones RN. Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America: SENTRY Antimicrobial Surveillance Program, 1997–2000. Int J Antimicrob Agents. 2002;20(6):412–8. Epub 2002/11/30. . - PubMed
-
- Lesens O, Hansmann Y, Brannigan E, Remy V, Hopkins S, Martinot M, et al. Positive surveillance blood culture is a predictive factor for secondary metastatic infection in patients with Staphylococcus aureus bacteraemia. J Infect. 2004;48(3):245–52. Epub 2004/03/06. 10.1016/j.jinf.2003.10.010 . - DOI - PubMed
-
- Ringberg H, Thoren A, Lilja B. Metastatic complications of Staphylococcus aureus septicemia. To seek is to find. Infection. 2000;28(3):132–6. Epub 2000/07/06. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

