Does canine inflammatory bowel disease influence gut microbial profile and host metabolism?
- PMID: 27306031
- PMCID: PMC4910228
- DOI: 10.1186/s12917-016-0736-2
Does canine inflammatory bowel disease influence gut microbial profile and host metabolism?
Abstract
Background: Inflammatory bowel disease (IBD) refers to a diverse group of chronic gastrointestinal diseases, and gut microbial dysbiosis has been proposed as a modulating factor in its pathogenesis. Several studies have investigated the gut microbial ecology of dogs with IBD but it is yet unclear if this microbial profile can alter the nutrient metabolism of the host. The aim of the present study was to characterize the faecal bacterial profile and functionality as well as to determine host metabolic changes in IBD dogs. Twenty-three dogs diagnosed with IBD and ten healthy control dogs were included. Dogs with IBD were given a clinical score using the canine chronic enteropathy clinical activity index (CCECAI). Faecal short-chain fatty acids (SCFA) and ammonia concentrations were measured and quantitative PCR was performed. The concentration of plasma amino acids, acylcarnitines, serum folate, cobalamin, and indoxyl sulfate was determined.
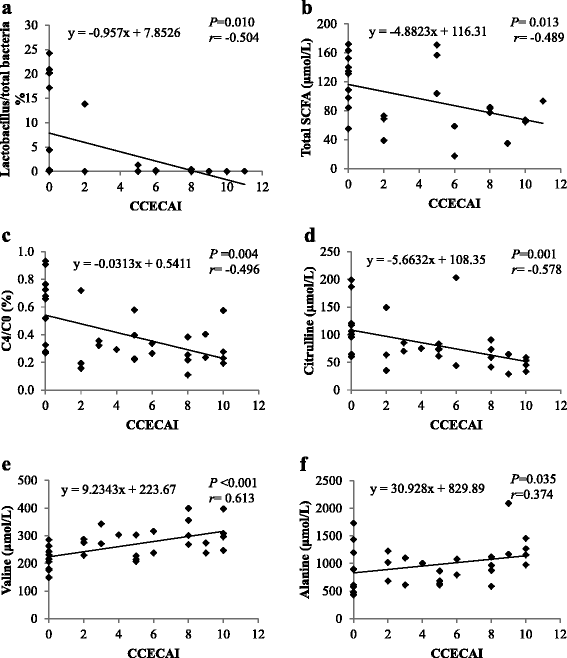
Results: No significant differences in the abundance of a selection of bacterial groups and fermentation metabolites were observed between the IBD and control groups. However, significant negative correlations were found between CCECAI and the faecal proportion of Lactobacillus as well as between CCECAI and total SCFA concentration. Serum folate and plasma citrulline were decreased and plasma valine was increased in IBD compared to control dogs. Increased plasma free carnitine and total acylcarnitines were observed in IBD compared with control dogs, whereas short-chain acylcarnitines (butyrylcarnitine + isobutyrylcarnitine and, methylmalonylcarnitine) to free carnitine ratios decreased. Dogs with IBD had a higher 3-hydroxyisovalerylcarnitine + isovalerylcarnitine to leucine ratio compared to control dogs.
Conclusions: Canine IBD induced a wide range of changes in metabolic profile, especially for the plasma concentrations of short-chain acylcarnitines and amino acids, which could have evolved from tissue damage and alteration in host metabolism. In addition, dogs with more severe IBD were characterised by a decrease in faecal proportion of Lactobacillus.
Keywords: Acylcarnitine profile; Butyrate-producing bacteria; Citrulline; Dog; Fermentation; Inflammatory bowel disease; Lactobacillus; Microbiota; Short-chain fatty acid.
Figures
Similar articles
-
The response of canine faecal microbiota to increased dietary protein is influenced by body condition.BMC Vet Res. 2017 Dec 4;13(1):374. doi: 10.1186/s12917-017-1276-0. BMC Vet Res. 2017. PMID: 29202841 Free PMC article.
-
Plasma amino acid profiles in dogs with inflammatory bowel disease.J Vet Intern Med. 2019 Jul;33(4):1602-1607. doi: 10.1111/jvim.15525. Epub 2019 May 20. J Vet Intern Med. 2019. PMID: 31111561 Free PMC article.
-
Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease.Gut Microbes. 2015;6(1):33-47. doi: 10.1080/19490976.2014.997612. Epub 2015 Jan 7. Gut Microbes. 2015. PMID: 25531678 Free PMC article.
-
METABOLIC DYSBIOSIS OF THE GUT MICROBIOTA AND ITS BIOMARKERS.Eksp Klin Gastroenterol. 2016 Jul;12(12):6-29. Eksp Klin Gastroenterol. 2016. PMID: 29889418 Review. English, Russian.
-
Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs.World J Gastroenterol. 2014 Nov 28;20(44):16489-97. doi: 10.3748/wjg.v20.i44.16489. World J Gastroenterol. 2014. PMID: 25469017 Free PMC article. Review.
Cited by
-
Comparison of the Effects of Enzymolysis Seaweed Powder and Saccharomyces boulardii on Intestinal Health and Microbiota Composition in Kittens.Metabolites. 2023 May 8;13(5):637. doi: 10.3390/metabo13050637. Metabolites. 2023. PMID: 37233678 Free PMC article.
-
Catching a glimpse of the bacterial gut community of companion animals: a canine and feline perspective.Microb Biotechnol. 2020 Nov;13(6):1708-1732. doi: 10.1111/1751-7915.13656. Epub 2020 Aug 30. Microb Biotechnol. 2020. PMID: 32864871 Free PMC article. Review.
-
Nonpharmacological Treatment Strategies for the Management of Canine Chronic Inflammatory Enteropathy-A Narrative Review.Vet Sci. 2022 Jan 20;9(2):37. doi: 10.3390/vetsci9020037. Vet Sci. 2022. PMID: 35202290 Free PMC article. Review.
-
Causal relationship between human blood metabolites and risk of ischemic stroke: a Mendelian randomization study.Front Genet. 2024 Jan 19;15:1333454. doi: 10.3389/fgene.2024.1333454. eCollection 2024. Front Genet. 2024. PMID: 38313676 Free PMC article.
-
Assessing the causal relationship between metabolic biomarkers and coronary artery disease by Mendelian randomization studies.Sci Rep. 2024 Aug 16;14(1):19034. doi: 10.1038/s41598-024-69879-2. Sci Rep. 2024. PMID: 39152174 Free PMC article.
References
-
- Rossi G, Pengo G, Caldin M, Palumbo Piccionello A, Steiner JM, Cohen ND, et al. Comparison of Microbiological, Histological, and Immunomodulatory Parameters in Response to Treatment with Either Combination Therapy with Prednisone and Metronidazole or Probiotic VSL#3 Strains in Dogs with Idiopathic Inflammatory Bowel Disease. Plos One. 2014;9(4):e94699. doi: 10.1371/journal.pone.0094699. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

